N-ethylacetamide
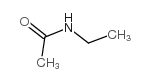
N-ethylacetamide structure
|
Common Name | N-ethylacetamide | ||
|---|---|---|---|---|
| CAS Number | 625-50-3 | Molecular Weight | 87.12040 | |
| Density | 0.924 g/mL at 25 °C(lit.) | Boiling Point | 90-92 °C8 mm Hg(lit.) | |
| Molecular Formula | C4H9NO | Melting Point | -32°C | |
| MSDS | Chinese USA | Flash Point | 224 °F | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Shear and dielectric responses of propylene carbonate, tripropylene glycol, and a mixture of two secondary amides.
J. Chem. Phys. 137(6) , 064508, (2012) Propylene carbonate and a mixture of two secondary amides, N-methylformamide and N-ethylacetamide, are investigated by means of broadband dielectric and mechanical shear spectroscopy. The similarities between the rheological and the dielectric responses of th... |
|
|
Acetyl Methyl Torsion in N-Ethylacetamide: A Challenge for Microwave Spectroscopy and Quantum Chemistry.
ChemPhysChem 16 , 1906-11, (2015) The gas-phase structures and parameters describing acetyl methyl torsion of N-ethylacetamide are determined with high accuracy, using a combination of molecular beam Fourier-transform microwave spectroscopy and quantum chemical calculations. Conformational st... |
|
|
The effect of anesthetics on hydrogen bonds. An infrared study at low anesthetic concentrations.
Biophys. Chem. 22(4) , 249-54, (1985) It is shown that a striking parallelism exists between the anesthetic potency of general halocarbon anesthetics and their influence on the hydrogen bond association constants in N-H...O=C type hydrogen bonds, important for shaping the ion channels. It is furt... |
|
|
Identification of dielectric and structural relaxations in glass-forming secondary amides.
J. Chem. Phys. 123(5) , 054516, (2005) Dielectric relaxation dynamics of secondary amides is explored in their supercooled state near the glass transition temperature Tg by investigating N-ethylacetamide and its mixtures with N-methylformamide. All the samples are found to exhibit giant dielectric... |
|
|
Fractional Stokes-Einstein-Debye relation and orientational entropy effects in strongly hydrogen-bonded liquid amides.
Phys. Chem. Chem. Phys. 13(9) , 3911-6, (2011) The impedance spectroscopy studies performed for two strongly hydrogen-bonded liquid amides: N-methylpropionamide (NMP, CH(3)·NH·CO·C(2)H(5)) and N-ethylacetamide (NEA, C(2)H(5)·NH·CO·CH(3)) have shown that the two centers of the peptide linkage, -NH·CO-, act... |
|
|
Pentacyclic polyketides from Endiandra kingiana as inhibitors of the Bcl-xL/Bak interaction.
Phytochemistry 72(11-12) , 1443-52, (2011) An in vitro biological screening of Malaysian plants allowed the selection of several species with a significant binding affinity for the antiapoptotic protein Bcl-xL. The chemical investigation of Endiandra kingiana led to the isolation of a series of polyke... |
|
|
Involvement of basal protein kinase C and extracellular signal-regulated kinase 1/2 activities in constitutive internalization of AMPA receptors in cerebellar Purkinje cells.
J. Neurosci. 26(18) , 4820-5, (2006) AMPA receptor (AMPAR) internalization provides a mechanism for long-term depression (LTD) in both hippocampal pyramidal neurons and cerebellar Purkinje cells (PCs). Cerebellar LTD at the parallel fiber (PF)-PC synapse is the underlying basis of motor learning... |
|
|
Photostability of Anthraquinone and Azo Dyes in N-Ethylacetamide (Nylon Model). Chang IY and Miller IK.
J. Soc. Dyers Colourists 102(2) , 46-53, (1986)
|
|
|
New On-line Mass Spectrometer for Identification of Reaction Products in the Aqueous Phase: Application to the OH-oxidation of N-methylpyrrolidone under Atmospheric Conditions. Poulain L, et al.
Environmental Simulation Chambers: Application to Atmospheric Chemical Processes Netherlands , (2006), 83-96
|