| Structure | Name/CAS No. | Articles |
|---|---|---|
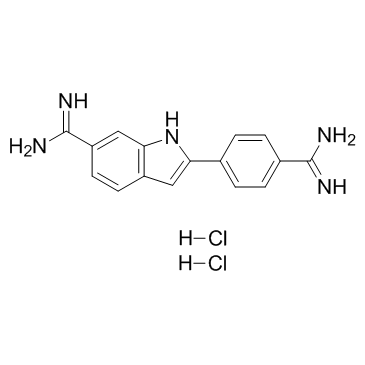 |
4',6-Diamidino-2-phenylindole dihydrochloride
CAS:28718-90-3 |
|
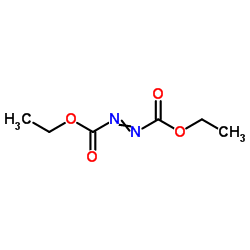 |
dead
CAS:1972-28-7 |
|
 |
Defensin HNP-1 human
CAS:99287-08-8 |