LNA guanine and 2,6-diaminopurine. Synthesis, characterization and hybridization properties of LNA 2,6-diaminopurine containing oligonucleotides.
Christoph Rosenbohm, Daniel Sejer Pedersen, Miriam Frieden, Flemming R Jensen, Susan Arent, Sine Larsen, Troels Koch
Index: Bioorg. Med. Chem. 12 , 2385-2396, (2004)
Full Text: HTML
Abstract
LNA guanine and 2,6-diaminopurine (D) phosphoramidites have been synthesized as building blocks for antisense oligonucleotides (ON). The effects of incorporating LNA D into ON were investigated. As expected, LNA D containing ON showed increased affinity towards complementary DNA (Delta Tm +1.6 to +3.0 degrees C) and RNA (Delta Tm +2.6 to +4.6 degrees C) ON. To evaluate if LNA D containing ON have an enhanced mismatch sensitivity compared to their complementary LNA A containing ON thermal denaturation experiments towards singly mismatched DNA and RNA ON were undertaken. Replacing one LNA A residue with LNA D, in fully LNA modified ON, resulted in higher mismatch sensitivity towards DNA ON (Delta Delta Tm -4 to >-17 degrees C). The same trend was observed towards singly mismatched RNA ON (Delta Delta Tm D-a = -8.7 degrees C and D-g = -4.5 degrees C) however, the effect was less clearcut and LNA A showed a better mismatch sensitivity than LNA D towards cytosine (Delta Tm +5.5 degrees C).
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
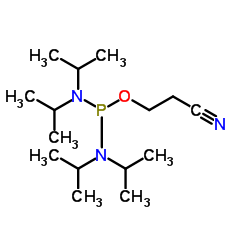 |
2-Cyanoethyltetraisopropylphosphorodiamidite
CAS:102691-36-1 |
C15H32N3OP |
|
2'-fluoro-4'-thioarabino-modified oligonucleotides: conforma...
2007-01-01 [Nucleic Acids Res. 35(5) , 1441-51, (2007)] |
|
DNA adducts of acrolein: site-specific synthesis of an oligo...
2002-05-01 [Chem. Res. Toxicol. 15(5) , 607-13, (2002)] |
|
Synthesis and fluorescence studies of multiple labeled oligo...
2004-01-01 [Bioconjug. Chem. 15 , 638-646, (2004)] |
|
Application of 2-cyanoethyl N,N,N',N'-tetraisopropylphosphor...
1986-09-25 [Nucleic Acids Res. 14(18) , 7391-403, (1986)] |
|
Synthesis of 1,2-diacyl-sn-glycerophosphatidylserine from eg...
1996-05-01 [Lipids 31(5) , 541-6, (1996)] |