Acetaminophen-induced oxidation of protein thiols. Contribution of impaired thiol-metabolizing enzymes and the breakdown of adenine nucleotides.
M A Tirmenstein, S D Nelson
Index: J. Biol. Chem. 265(6) , 3059-65, (1990)
Full Text: HTML
Abstract
The administration of a hepatotoxic dose of acetaminophen (250 mg/kg) to mice induced the loss of protein thiols in mouse liver. Our data suggest that a significant portion of this loss was due to protein thiol oxidation. The administration of the nonhepatotoxic regioisomer, 3'-hydroxyacetanilide (600 mg/kg) did not produce a similar decrease in liver protein thiols despite producing similar levels of covalent binding. Mice treated with acetaminophen exhibited decreased glutathione peroxidase activity, decreased thioltransferase activity, and decreased adenine nucleotide concentrations in the liver. The increase in urinary allantoin after the administration of acetaminophen suggests that the decrease in adenine nucleotides was due to their degradation in the liver. Acetaminophen also promoted the conversion of the enzyme xanthine dehydrogenase to the oxidase form, and pretreatment of mice with allopurinol, an inhibitor of xanthine oxidase, significantly decreased acetaminophen-mediated hepatotoxicity. The conversion of xanthine dehydrogenase to the oxidase form may lead to a transient increase in the production of activated oxygen species. The increase in activated oxygen species coupled with decreases in glutathione peroxidase and thioltransferase activity may be responsible in part for the increased levels of oxidized protein thiols observed following acetaminophen administration.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
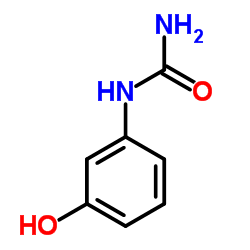 |
1-(3-Hydroxyphenyl)urea
CAS:621-42-1 |
C8H9NO2 |
|
Developing structure-activity relationships for the predicti...
2010-07-19 [Chem. Res. Toxicol. 23 , 1215-22, (2010)] |
|
A predictive ligand-based Bayesian model for human drug-indu...
2010-12-01 [Drug Metab. Dispos. 38 , 2302-8, (2010)] |
|
Cellular apoptosis and cytotoxicity of phenolic compounds: a...
2005-11-17 [J. Med. Chem. 48 , 7234-42, (2005)] |
|
Favipiravir inhibits acetaminophen sulfate formation but min...
2015-11-01 [Br. J. Clin. Pharmacol. 80 , 1076-85, (2015)] |
|
Use of a systems model of drug-induced liver injury (DILIsym...
2014-04-21 [Toxicol. Lett. 226(2) , 163-72, (2014)] |