1-(3-Hydroxyphenyl)urea
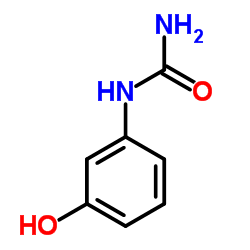
1-(3-Hydroxyphenyl)urea structure
|
Common Name | 1-(3-Hydroxyphenyl)urea | ||
|---|---|---|---|---|
| CAS Number | 621-42-1 | Molecular Weight | 152.151 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 313.1±25.0 °C at 760 mmHg | |
| Molecular Formula | C8H9NO2 | Melting Point | 145-148 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 143.2±23.2 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Developing structure-activity relationships for the prediction of hepatotoxicity.
Chem. Res. Toxicol. 23 , 1215-22, (2010) Drug-induced liver injury is a major issue of concern and has led to the withdrawal of a significant number of marketed drugs. An understanding of structure-activity relationships (SARs) of chemicals can make a significant contribution to the identification o... |
|
|
A predictive ligand-based Bayesian model for human drug-induced liver injury.
Drug Metab. Dispos. 38 , 2302-8, (2010) Drug-induced liver injury (DILI) is one of the most important reasons for drug development failure at both preapproval and postapproval stages. There has been increased interest in developing predictive in vivo, in vitro, and in silico models to identify comp... |
|
|
Cellular apoptosis and cytotoxicity of phenolic compounds: a quantitative structure-activity relationship study.
J. Med. Chem. 48 , 7234-42, (2005) In this comprehensive study on the caspase-mediated apoptosis-inducing effect of 51 substituted phenols in a murine leukemia cell line (L1210), we determined the concentrations needed to induce caspase activity by 50% (I50) and utilized these data to develop ... |
|
|
Favipiravir inhibits acetaminophen sulfate formation but minimally affects systemic pharmacokinetics of acetaminophen.
Br. J. Clin. Pharmacol. 80 , 1076-85, (2015) The antiviral agent favipiravir is likely to be co-prescribed with acetaminophen (paracetamol). The present study evaluated the possiblility of a pharmacokinetic interaction between favipiravir and acetaminophen, in vitro and in vivo.The effect of favipivir o... |
|
|
Use of a systems model of drug-induced liver injury (DILIsym®) to elucidate the mechanistic differences between acetaminophen and its less-toxic isomer, AMAP, in mice
Toxicol. Lett. 226(2) , 163-72, (2014) Acetaminophen (APAP) has been used as a probe drug to investigate drug-induced liver injury (DILI). In mice, 3′-hydroxyacetanilide (AMAP), a less-toxic isomer of APAP, has also been studied as a negative control. Various mechanisms for the divergence in toxic... |
|
|
The metabolism of 2- and 3-hydroxyacetanilide. Determination of metabolic products by liquid chromatography/electrochemistry.
Drug Metab. Dispos. 14(1) , 5-12, (1986) The metabolism of 2- and 3-hydroxyacetanilide was examined in incubations with mouse liver microsomes. Metabolic products were determined by a comparison of both chromatographic and electrochemical properties with that of reference materials. Using this techn... |
|
|
Cell culture model for acetaminophen-induced hepatocyte death in vivo.
Biochem. Pharmacol. 64(3) , 413-24, (2002) Overdose of the popular, and relatively safe, analgesic acetaminophen (N-acetyl-p-aminophenol, APAP, paracetamol) can produce a fatal centrilobular liver injury. APAP-induced cell death was investigated in a differentiated, transforming growth factor alpha (T... |
|
|
Hepatic protein arylation, glutathione depletion, and metabolite profiles of acetaminophen and a non-hepatotoxic regioisomer, 3'-hydroxyacetanilide, in the mouse.
Drug Metab. Dispos. 18(5) , 765-70, (1990) The metabolism and disposition of acetaminophen (APAP) and a non-hepatotoxic regioisomer, 3'-hydroxyacetanilide (AMAP), were investigated in the mouse using 14C-labeled analogues. Covalent binding of metabolites of both compounds was observed on the order of ... |
|
|
Acetaminophen-induced oxidation of protein thiols. Contribution of impaired thiol-metabolizing enzymes and the breakdown of adenine nucleotides.
J. Biol. Chem. 265(6) , 3059-65, (1990) The administration of a hepatotoxic dose of acetaminophen (250 mg/kg) to mice induced the loss of protein thiols in mouse liver. Our data suggest that a significant portion of this loss was due to protein thiol oxidation. The administration of the nonhepatoto... |
|
|
Metabolic alterations resulting from the inhibition of mitochondrial respiration by acetaminophen in vivo.
Biochem. Pharmacol. 38(14) , 2390-2, (1989)
|