N-heterocycle carbene (NHC)-ligated cyclopalladated N,N-dimethylbenzylamine: a highly active, practical and versatile catalyst for the Heck-Mizoroki reaction.
Guang-Rong Peh, Eric Assen B Kantchev, Chi Zhang, Jackie Y Ying
Index: Org. Biomol. Chem. 7(10) , 2110-9, (2009)
Full Text: HTML
Abstract
The wide dissemination of catalytic protocols in academic and industrial laboratories is facilitated by the development of catalysts that are not only highly active but also user-friendly, stable to moisture, air and long term storage and easy to prepare on a large scale. Herein we describe a protocol for the Heck-Mizoroki reaction mediated by cyclopalladated N,N-dimethylbenzylamine (dmba) ligated with a N-heterocyclic carbene, 1,3-bis(mesityl)imidazol-2-ylidene (IMes), that fulfils these criteria. The precatalyst can be synthesized on approximately 100 g scale by a tri-component, sequential, one-pot reaction of N,N-dimethylbenzylamine, PdCl2 and IMes.HCl in refluxing acetonitrile in air in the presence of K2CO3. This single component catalyst is stable to air, moisture and long term storage and can be conveniently dispensed as a stock solution in NMP. It mediates the Heck-Mizoroki reaction of a range of aryl- and heteroaryl bromides in reagent grade NMP at the 0.1-2 mol% range without the need for rigorous anhydrous techniques or a glovebox, and is active even in air. The catalyst is capable of achieving very high levels of catalytic activity (TON of up to 5.22 x 10(5)) for the coupling of a deactivated arylbromide, p-bromoanisole, with tBu acrylate as a benchmark substrate pair. A wide range of aryl bromides, iodides and, for the first time with a NHC-Pd catalyst, a triflate was coupled with diverse acrylate derivatives (nitrile, tert-butyl ester and amides) and styrene derivatives. The use of excess (>2 equiv.) of the aryl bromide and tert-butyl acrylate leads to mixture of tert-butyl beta,beta-diarylacrylate and tert-butyl cinnamate derivatives depending on the substitution pattern of the aryl bromide. Electron rich m- and p-substituted arylbromides give the diarylated products exclusively, whereas electron-poor aryl bromides give predominantly mono-arylated products. For o-substituted aryl bromides, no doubly arylated products could be obtained under any conditions. Overall, the active catalyst (IMes-Pd) shows higher activity with electron-rich aryl halides, a marked difference compared with the more commonly used phosphane-Pd or non-ligated Pd catalysts.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
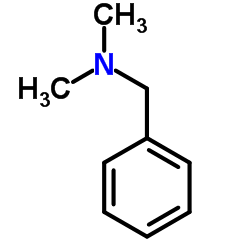 |
dimethylbenzylamine
CAS:103-83-3 |
C9H13N |
|
Silver nanowire interactions with primary human alveolar typ...
2015-06-21 [Nanoscale 7 , 10398-409, (2015)] |
|
Lipophilicity of basic drugs measured by hydrophilic interac...
2009-05-28 [J. Med. Chem. 52 , 3416-9, (2009)] |
|
Benzylamine analog binding studies as probes of the substrat...
1999-02-01 [Drug Metab. Rev. 31(1) , 235-45, (1999)] |
|
Designing multi-viscosity embedding media utilizing novel re...
1992-01-01 [Microsc. Res. Tech. 20(1) , 105-6, (1992)] |
|
Aqueous solubility study of salts of benzylamine derivatives...
2004-01-09 [Int. J. Pharm. 269(1) , 157-68, (2004)] |