| Structure | Name/CAS No. | Articles |
|---|---|---|
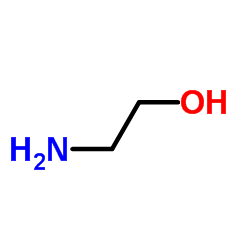 |
2-Aminoethanol
CAS:141-43-5 |
|
 |
L-Nicotine
CAS:54-11-5 |
|
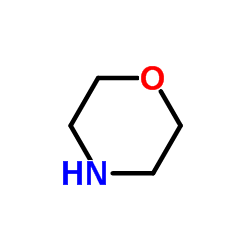 |
morpholine
CAS:110-91-8 |
|
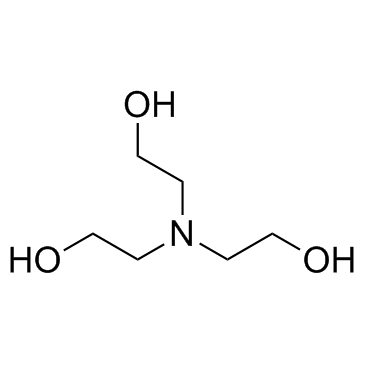 |
Triethanolamine
CAS:102-71-6 |
|
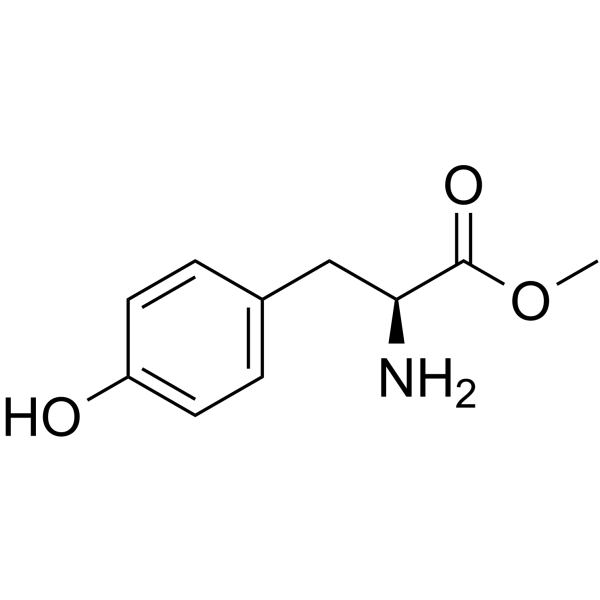 |
H-Tyr-OMe
CAS:1080-06-4 |
|
 |
Lidocaine
CAS:137-58-6 |
|
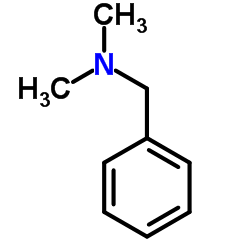 |
dimethylbenzylamine
CAS:103-83-3 |
|
 |
carazolol
CAS:57775-29-8 |
|
 |
Atenolol
CAS:29122-68-7 |
|
 |
pseudoephedrine
CAS:90-82-4 |