Enantioselective synthesis of tryptophan derivatives by a tandem Friedel-Crafts conjugate addition/asymmetric protonation reaction.
Madeleine E Kieffer, Lindsay M Repka, Sarah E Reisman
Index: J. Am. Chem. Soc. 134(11) , 5131-7, (2012)
Full Text: HTML
Abstract
The tandem Friedel-Crafts conjugate addition/asymmetric protonation reaction between 2-substituted indoles and methyl 2-acetamidoacrylate is reported. The reaction is catalyzed by (R)-3,3'-dibromo-BINOL in the presence of stoichiometric SnCl(4), and is the first example of a tandem conjugate addition/asymmetric protonation reaction using a BINOL·SnCl(4) complex as the catalyst. A range of indoles furnished synthetic tryptophan derivatives in good yields and high levels of enantioselectivity, even on a preparative scale. The convergent nature of this transformation should lend itself to the preparation of unnatural tryptophan derivatives for use in a broad array of synthetic and biological applications.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
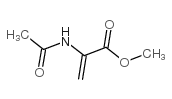 |
Methyl 2-acetamidoacrylate
CAS:35356-70-8 |
C6H9NO3 |
|
The ethyl pyruvate analogues, diethyl oxaloproprionate, 2-ac...
2005-11-25 [Biochem. Pharmacol. 70(11) , 1579-92, (2005)] |
|
Methyl-2-acetamidoacrylate, an ethyl pyruvate analog, decrea...
2008-12-01 [Am. J. Physiol. Renal Physiol. 295(6) , F1825-35, (2008)] |
|
Orthogonal synthesis of indolines and isoquinolines via aryn...
2008-02-06 [J. Am. Chem. Soc. 130(5) , 1558-9, (2008)] |
|
Asymmetric hydrogenation using chiral Rh complexes immobilis...
2005-04-21 [Org. Biomol. Chem. 3(8) , 1547-50, (2005)] |
|
Extended para-hydrogenation monitored by NMR spectroscopy.
2011-01-21 [Chem. Commun. (Camb.) 47(3) , 958-60, (2011)] |