Separation of chiral primary amino compounds by forming a sandwiched complex in reversed-phase high performance liquid chromatography.
Chen Zhang, Wei X Huang, Zhi Chen, Abu M Rustum
Index: J. Chromatogr. A. 1217(30) , 4965-70, (2010)
Full Text: HTML
Abstract
Separation of chiral primary amino compounds was efficiently achieved under reversed-phase high performance liquid chromatography (RP-HPLC) conditions using a mixture of non-chiral crown ether (18-crown-6) and dimethyl-beta-cyclodextrin (DM-beta-CD) in the mobile phase. Under these conditions, the amino group of the chiral compound was protonated in a low pH mobile phase, and then interacted with 18-crown-6 and DM-beta-CD to form a sandwiched complex [18-crown-6+amine+CD]. Enantiomers of the compound in the sandwiched complex were separated with good enantioselectivity. Formation of the sandwiched complex among the chiral compound and additives in the mobile phase is a key step of the chiral separation. Four different chiral amino compounds namely, 1-aminoindan (AI), 1,2,3,4-tetrahydro-1-naphthylamine (THNA), tyrosine (Tyr), and phenylalanine (Phe), were selected to demonstrate the separation using the sandwiched complex mechanism in RP-HPLC.Copyright (c) 2010 Elsevier B.V. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
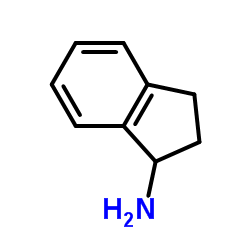 |
1-Indanamine
CAS:34698-41-4 |
C9H11N |
|
A colorimetric chiral sensor based on chiral crown ether for...
2011-04-01 [Chirality 23(4) , 349-53, (2011)] |
|
Laser-induced fluorescence and single vibronic level emissio...
2006-02-28 [Phys. Chem. Chem. Phys. 8(8) , 1001-6, (2006)] |
|
Oxygen-18 studies on the oxidative deamination mechanism of ...
1982-05-01 [Arch. Biochem. Biophys. 215(2) , 433-43, (1982)] |
|
Low-frequency vibrations specific for conformers of 1-aminoi...
2007-06-07 [J. Chem. Phys. 126(21) , 214304, (2007)] |
|
Rapid enantioseparation of 1-aminoindan by microchip electro...
2005-01-01 [Anal. Sci. 21(1) , 61-5, (2005)] |