Carbon monoxide and nitric oxide modulate α₂-antiplasmin and plasmin activity: role of heme.
Matthew R Arkebauer, Sri S Kanaparthy, Saninuj N Malayaman, Keith Vosseller, Vance G Nielsen
Index: Blood Coagul. Fibrinolysis 22(8) , 712-9, (2011)
Full Text: HTML
Abstract
Cigarette smoke and carbon monoxide (CO) released from tricarbonyldichlororuthenium (II) dimer (CORM-2) attenuate fibrinolysis. The purpose of the present study was to determine whether CO diminished fibrinolysis by enhancement of α₂-antiplasmin via a putative heme group. Plasma, isolated α₂-antiplasmin and isolated plasmin were exposed to CO released from CORM-2 and nitric oxide (NO) via a NO donor to induce carboxyheme and metheme states, respectively. Exposed, isolated enzymes were placed in either α₂-antiplasmin-deficient or normal plasma. Effects of CO and NO on tissue-type plasminogen activator initiated fibrinolysis were determined by thrombelastography. Liquid chromatography-mass spectrometry (LC-MS/MS) was used to identify heme released from α₂-antiplasmin and plasmin. CO significantly enhanced α₂-antiplasmin activity, but decreased plasmin activity. NO decreased both α₂-antiplasmin and plasmin activity. Although inadequate LC-MS/MS data were obtained with α₂-antiplasmin (secondary to glycosylation), a putative plasmin-associated heme was identified. CO elicits hypofibrinolysis by enhancing α₂-antiplasmin activity and decreasing plasmin activity. On the basis of the responses to NO and LC-MS/MS data, it is highly likely that both enzymes are modulated by attached heme groups. Efforts to develop methods to detect CO-mediated hypercoagulability are ongoing, with the goal of identifying populations at risk of thrombotic morbidity secondary to cigarette smoking.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
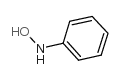 |
N-Phenylhydroxylamine
CAS:100-65-2 |
C6H7NO |
|
In situ infrared monitoring of the solid/liquid catalyst int...
2011-07-21 [Phys. Chem. Chem. Phys. 13(27) , 12463-71, (2011)] |
|
Genotoxic activities of aniline and its metabolites and thei...
2005-12-01 [Crit. Rev. Toxicol. 35(10) , 783-835, (2005)] |
|
Biotransformation of hydroxylaminobenzene and aminophenol by...
2000-06-01 [Appl. Environ. Microbiol. 66(6) , 2336-42, (2000)] |
|
Superoxide dismutase-mediated reversible conversion of 3-hyd...
1988-11-01 [Carcinogenesis 9(11) , 2003-8, (1988)] |
|
[Phenoxenium ions: generations and reactions].
1994-08-01 [Yakugaku Zasshi 114(8) , 565-76, (1994)] |