| Structure | Name/CAS No. | Articles |
|---|---|---|
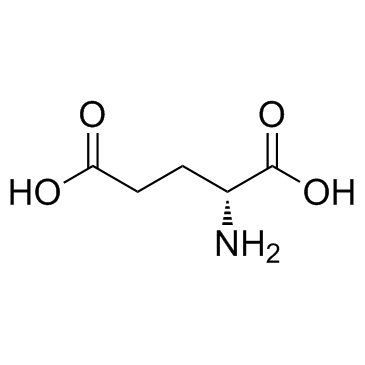 |
D(-)-Glutamic acid
CAS:6893-26-1 |
|
 |
α-Aminoadipic acid
CAS:1118-90-7 |