Ascorbic acid reduction of microtubule protein disulfides and its relevance to protein S-nitrosylation assays.
Lisa M Landino, Maria T Koumas, Courtney E Mason, Jane A Alston
Index: Biochem. Biophys. Res. Commun. 340(2) , 347-52, (2006)
Full Text: HTML
Abstract
The biotin switch assay was developed to aid in the identification of S-nitrosylated proteins in different cell types. However, our work with microtubule proteins including tubulin and its associated proteins tau and microtubule-associated protein-2 shows that ascorbic acid is not a selective reductant of protein S-nitrosothiols as described in the biotin switch assay. Herein we show that ascorbic acid reduces protein disulfides in tubulin, tau, and microtubule-associated protein-2 that are formed by peroxynitrite anion. Reduction of microtubule-associated protein disulfides by ascorbic acid following peroxynitrite treatment restores microtubule polymerization kinetics to control levels. We also show that ascorbic acid reduces the disulfide dithiobis(2-nitrobenzoic acid), a reagent commonly used to detect protein thiols. Not only do we describe a new reactivity of ascorbic acid with microtubule proteins but we expose an important limitation when using the biotin switch assay to detect protein S-nitrosylation.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
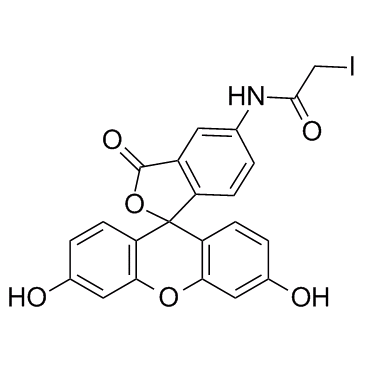 |
5-(Iodoacetamido)fluorescein
CAS:63368-54-7 |
C22H14INO6 |
|
Combination of direct infusion mass spectrometry and gas chr...
2015-03-15 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 985 , 75-84, (2015)] |
|
Optical control of protein-protein interactions via blue lig...
2014-08-05 [Biochemistry 53(30) , 5008-16, (2014)] |
|
Redox signalling to nuclear regulatory proteins by reactive ...
2015-05-12 [Br. J. Cancer 112 , 1687-702, (2015)] |
|
Analysis of alpha-1-acid glycoprotein isoforms using CE-LIF ...
2012-04-01 [Electrophoresis 33(7) , 1113-9, (2012)] |
|
Impact of glutathione on the allergenicity of the peach lipi...
2015-01-01 [J. Investig. Allergol. Clin. Immunol. 25(1) , 47-54, (2015)] |