Organocatalytic asymmetric epoxidation and tandem epoxidation/Passerini reaction under eco-friendly reaction conditions.
Anna Maria Deobald, Arlene G Corrêa, Daniel G Rivera, Márcio Weber Paixão
Index: Org. Biomol. Chem. 10(38) , 7681-4, (2012)
Full Text: HTML
Abstract
An eco-friendly synthesis of highly functionalized epoxides and their incorporation into an organocatalytic multicomponent approach are reported. For this, a modified class of diarylprolinol silyl ethers was designed to enable high catalytic activity in an environmentally benign solvent system. The one-pot procedure showed great efficiency in promoting stereoselective multicomponent transformations in a tandem, 'green' fashion. Because of its non-residual, efficient and selective character, this synthetic design shows promise for large-scale applications in both diversity and target-oriented syntheses.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
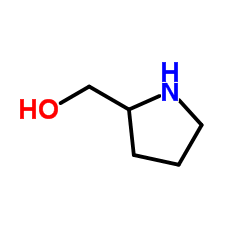 |
L-Pro-ol
CAS:23356-96-9 |
C5H11NO |
|
Three-dimensional quantitative structure-activity relationsh...
2011-11-01 [Bioorg. Med. Chem. 19 , 6409-18, (2011)] |
|
Chiral Diphenylprolinol TES ether promoted conjugate additio...
2007-03-15 [Org. Lett. 9(6) , 965-8, (2007)] |
|
Asymmetric alkynylation of aldehydes with propiolates withou...
2011-06-21 [Org. Biomol. Chem. 9(12) , 4425-8, (2011)] |
|
CBS reductions with a fluorous prolinol immobilized in a hyd...
2008-03-06 [Org. Lett. 10(5) , 749-52, (2008)] |
|
Access to optically active 3-azido- and 3-aminopiperidine de...
2011-08-19 [Org. Lett. 13(16) , 4442-5, (2011)] |