L-Pro-ol
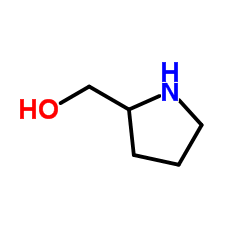
L-Pro-ol structure
|
Common Name | L-Pro-ol | ||
|---|---|---|---|---|
| CAS Number | 23356-96-9 | Molecular Weight | 101.147 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 211.0±0.0 °C at 760 mmHg | |
| Molecular Formula | C5H11NO | Melting Point | N/A | |
| MSDS | USA | Flash Point | 86.1±0.0 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of L-Pro-ol |
| Name | l-prolinol |
|---|---|
| Synonym | More Synonyms |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 211.0±0.0 °C at 760 mmHg |
| Molecular Formula | C5H11NO |
| Molecular Weight | 101.147 |
| Flash Point | 86.1±0.0 °C |
| Exact Mass | 101.084061 |
| PSA | 32.26000 |
| LogP | -0.67 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.455 |
| InChIKey | HVVNJUAVDAZWCB-YFKPBYRVSA-N |
| SMILES | OCC1CCCN1 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S37/39 |
| WGK Germany | 3 |
| HS Code | 2933990090 |
| Precursor 6 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Three-dimensional quantitative structure-activity relationship analyses of substrates of the human proton-coupled amino acid transporter 1 (hPAT1).
Bioorg. Med. Chem. 19 , 6409-18, (2011) The proton-coupled amino acid transporter hPAT1 has recently gained much interest due to its ability to transport small drugs thereby allowing their oral administration. A three-dimensional quantitati... |
|
|
Chiral Diphenylprolinol TES ether promoted conjugate addition-aldol-dehydration reactions between alpha,beta-unsaturated aldehydes and 2-N-protected amino benzaldehydes.
Org. Lett. 9(6) , 965-8, (2007) A conjugate addition-aldol-dehydration reaction of alpha,beta-unsaturated aldehydes with 2-N-protected amino benzaldehydes has been developed. The process is promoted by (S)-diphenylprolinol TES ether... |
|
|
Asymmetric alkynylation of aldehydes with propiolates without high reagent loading and any additives.
Org. Biomol. Chem. 9(12) , 4425-8, (2011) The asymmetric alkynylation of aliphatic and aromatic aldehydes with propiolates was mediated by dialkylzinc and a novel prolinol catalyst without high reagent loading and any additives, such as Ti(Oi... |
| 2-Pyrrolidinemethanol |
| EINECS 245-605-2 |
| H-L-PRO-OL |
| MFCD00005255 |
| (S)-(+)-Prolinol |
| 2-Pyrrolidinylmethanol |
| DL-Prolinol |
| Pyrrolidine, 2-(hydroxymethyl)- |
| 2-Pyrrolidinemethanol, (2S)- |
| (S)-(+)-2-(Hydroxymethyl)Pyrrolidine |
| (2S)-pyrrolidin-2-ylmethanol |
| L-Prolinol |
| (2S)-2-Pyrrolidinylmethanol |
| (S)-Pyrrolidin-2-ylmethanol |
| H-PROLINOL |
| pyrrolidin-2-ylmethanol |
| Prolinol |
| L-Pro-ol |
| rac-prolinol |
| (S)-Prolinol |
| (2S)-2-Pyrrolidinemethanol |
| (S)-(+)-2-Pyrrolidinemethanol |
| H-PRO-OL |
| L(+)-Prolinol |
| (2S)-prolinol |
 CAS#:147-85-3
CAS#:147-85-3 CAS#:98-79-3
CAS#:98-79-3 CAS#:854917-80-9
CAS#:854917-80-9 CAS#:142-47-2
CAS#:142-47-2 CAS#:62024-36-6
CAS#:62024-36-6![3-(6-oxo-5R-phenyl-5,6-dihydro-2H-[1,4]oxazin-3-yl)propanoic acid methyl ester Structure](https://image.chemsrc.com/caspic/368/171561-60-7.png) CAS#:171561-60-7
CAS#:171561-60-7![[1-(4-nitrophenyl)pyrrolidin-2-yl]methanol structure](https://image.chemsrc.com/caspic/212/105173-13-5.png) CAS#:105173-13-5
CAS#:105173-13-5 CAS#:460746-46-7
CAS#:460746-46-7 CAS#:108-94-1
CAS#:108-94-1 CAS#:146405-63-2
CAS#:146405-63-2![1-[(2S)-2-Pyrrolidinylmethyl]pyrrolidine structure](https://image.chemsrc.com/caspic/419/51207-66-0.png) CAS#:51207-66-0
CAS#:51207-66-0 CAS#:186202-18-6
CAS#:186202-18-6![furan-2-yl-[(2S)-2-(methoxymethyl)pyrrolidin-1-yl]methanone structure](https://image.chemsrc.com/caspic/125/183014-03-1.png) CAS#:183014-03-1
CAS#:183014-03-1 CAS#:182277-12-9
CAS#:182277-12-9![[(2S)-1-(4-bromohexa-2,4-dienyl)pyrrolidin-2-yl]methanol structure](https://image.chemsrc.com/caspic/342/195443-47-1.png) CAS#:195443-47-1
CAS#:195443-47-1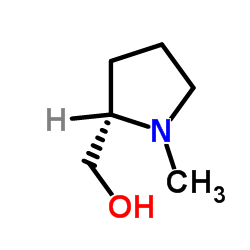 CAS#:34381-71-0
CAS#:34381-71-0
