Reaction of 3-deoxypentosulose with N-methyl- and N,N-dimethylguanidine as model reagents for protein-bound arginine and for creatine.
R Sopio, M Lederer
Index: Z. Lebensm. Unters. Forsch. 201(4) , 381-6, (1995)
Full Text: HTML
Abstract
Deoxyosones are established key-intermediates in Maillard processes. Due to their dicarbonyl structure, they undergo condensation to form heterocyclic compounds with guanidine derivatives. In biological systems, guanidino functions are present in protein-bound arginine moieties as well as in creatine. The reactivity of such structures towards 3-deoxypentosulose is investigated with N-methyl- and N,N-dimethylguanidine as model substrates. Two diastereoisomers each are isolated from both reactions; they have been characterized unequivocally, respectively, as 4-(2,3-dihydroxypropyl)-2-N-methylamino-2-imidazoline-5-one and 4-hydroxy-5-(2,3-dihydroxypropyl)-2-(N,N-dimethylamino)-5H-imidazole. In aqueous medium as well as in the crystalline state, both diastereoisomer pairs exist in different tautomeric forms.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
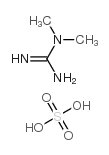 |
1,1-Dimethylguanidine sulfate
CAS:598-65-2 |
C3H11N3O4S |
|
Engineering of indole-based tethered biheterocyclic alkaloid...
2012-01-01 [Beilstein J. Org. Chem. 8 , 1901-8, (2012)] |
|
Interactions of guanidine and a related compound with potass...
1993-01-01 [Receptors Channels 1(3) , 181-91, (1993)] |
|
Effects of 1,1-dimethylguanidine administration on blood pre...
1997-01-01 [Acta Physiol. Scand. 159(1) , 1-6, (1997)] |
|
Inactivation of nitric oxide synthase isoforms by diaminogua...
1996-01-15 [Arch. Biochem. Biophys. 325(2) , 227-34, (1996)] |
|
Structure-activity relationship between guanidine alkyl deri...
1984-09-01 [J. Pharmacol. Exp. Ther. 230(3) , 710-7, (1984)] |