1,1-Dimethylguanidine sulfate
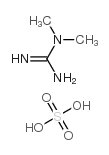
1,1-Dimethylguanidine sulfate structure
|
Common Name | 1,1-Dimethylguanidine sulfate | ||
|---|---|---|---|---|
| CAS Number | 598-65-2 | Molecular Weight | 185.20200 | |
| Density | N/A | Boiling Point | 104.7ºC at 760 mmHg | |
| Molecular Formula | C3H11N3O4S | Melting Point | 300 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 17.1ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Engineering of indole-based tethered biheterocyclic alkaloid meridianin into β-carboline-derived tetracyclic polyheterocycles via amino functionalization/6-endo cationic π-cyclization.
Beilstein J. Org. Chem. 8 , 1901-8, (2012) A mild, efficient and versatile method has been developed for the construction of a functionalized natural product, meridianin, and its post conversion to pyrimido-β-carboline by cationic π- cyclization. The strategy involves the introduction of an amino grou... |
|
|
Interactions of guanidine and a related compound with potassium channels in frog myelinated nerve fibre.
Receptors Channels 1(3) , 181-91, (1993) The effects of guanidine and dimethyl guanidine were studied on current- and voltage-clamped nodes of Ranvier, to determine the electrophysiological basis for the guanidine-induced increase in neurotransmitter release. When added to the external solution, gua... |
|
|
Effects of 1,1-dimethylguanidine administration on blood pressure, heart rate and renal sympathetic nerve activity in normotensive and spontaneously hypertensive rats.
Acta Physiol. Scand. 159(1) , 1-6, (1997) 1,1-dimethylguanidine (DMG) is an endogenous nitric oxide (NO) synthesis inhibitor. This study investigates the effects of exogenous DMG administration, in anaesthetized spontaneously hypertensive rats (SHR) and Wistar-Kyoto rats (WKY). Mean blood pressure (M... |
|
|
Reaction of 3-deoxypentosulose with N-methyl- and N,N-dimethylguanidine as model reagents for protein-bound arginine and for creatine.
Z. Lebensm. Unters. Forsch. 201(4) , 381-6, (1995) Deoxyosones are established key-intermediates in Maillard processes. Due to their dicarbonyl structure, they undergo condensation to form heterocyclic compounds with guanidine derivatives. In biological systems, guanidino functions are present in protein-boun... |
|
|
Inactivation of nitric oxide synthase isoforms by diaminoguanidine and NG-amino-L-arginine.
Arch. Biochem. Biophys. 325(2) , 227-34, (1996) Diaminoguanidine (DAG) and NG-amino-L-arginine each produced a time- and concentration-dependent inactivation of the citrulline-forming activity of all three NOS isoforms. DAG inactivates both the NADPH-oxidase and the citrulline-forming activities of GH3 pit... |
|
|
Structure-activity relationship between guanidine alkyl derivatives and norepinephrine release: site(s) and mechanism(s) of action.
J. Pharmacol. Exp. Ther. 230(3) , 710-7, (1984) The effect of guanidine alkyl derivatives on the evoked release of [3H]norepinephrine [( 3H]NE) from spleen strips was examined. Guanidine, methyl guanidine and N,N-dimethyl guanidine all enhanced the field-stimulated release of [3H]NE 2- to 3-fold, whereas N... |
|
|
Guanidine derivatives used as peroxide activators for bleaching cellulosic textiles. Cai JY and Evans DJ.
Color. Technol. 123(2) , 115-18, (2007)
|