A multiple-baseline, double-blind evaluation of the effects of trimeprazine tartrate on infant sleep disturbance.
K G France, N M Blampied, P Wilkinson
Index: Exp. Clin. Psychopharmacol. 7(4) , 502-13, (1999)
Full Text: HTML
Abstract
Infant sleep disturbance involving chronic night waking and resistance to settling to sleep or returning to sleep is a common problem for families with children 6-27 months old. Prescription and nonprescription sedatives are frequently administered without clear evidence that they are effective as either long-term or short-term palliatives. Trimeprazine tartrate, administered either 15 mg/5 mL or 30 mg/5 mL, was compared with both baseline and placebo in a multiple-baseline-across participants, double-blind study. No clinically significant effects of the low dose were detected, whereas the effects of the high dose were not consistently replicated across nor within participants. During active drug treatment, only 2 of 12 children achieved Sleep Behaviour Scale scores indicative of nonproblem sleep. Trimeprazine tartrate is not recommended as a pharmacological treatment for infant sleep disturbance unless as an adjunct to a behavioral therapy program.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
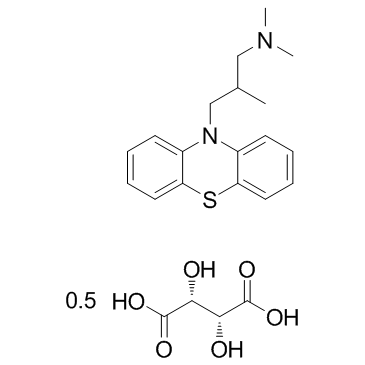 |
Alimemazine hemitartrate
CAS:4330-99-8 |
C18H22N2S.1/2C4H6O6 |
|
Alterations in intracranial pressure following ketamine anes...
1984-01-01 [Indian J. Pediatr. 51(408) , 25-8, (1984)] |
|
Pruritus in burns. Comments from the Vancouver General Hospi...
1988-01-01 [J. Burn Care Rehabil. 9(3) , 310-1, (1988)] |
|
Behavioural changes in children following minor surgery--is ...
1992-01-01 [Acta Anaesthesiol. Belg. 43(3) , 173-9, (1992)] |
|
A double-blind drug trial of treatment in young children wit...
1985-07-01 [J. Child Psychol. Psychiatry. 26(4) , 591-8, (1985)] |
|
Potentiation of radiation response of a mouse fibrosarcoma b...
1984-06-01 [Indian J. Exp. Biol. 22(6) , 305-7, (1984)] |