Phase II randomized study of dose-dense docetaxel and cisplatin every 2 weeks with pegfilgrastim and darbepoetin alfa with and without the chemoprotector BNP7787 in patients with advanced non-small cell lung cancer (CALGB 30303).
Antonius A Miller, Xiaofei F Wang, Lin Gu, Philip Hoffman, Jamil Khatri, Frank Dunphy, Martin J Edelman, Michael Bolger, Everett E Vokes, Mark R Green
Index: J. Thorac. Oncol. 3(10) , 1159-65, (2008)
Full Text: HTML
Abstract
We investigated dose-dense docetaxel and cisplatin in patients with measurable non-small cell lung cancer in a randomized phase II study without [A] or with [B] a putative chemoprotective agent, BNP7787.Chemotherapy-naive patients with stage IIIB (effusion) or IV, performance status 0 to 1, and adequate organ function were eligible. Treatment with docetaxel 75 mg/m followed by cisplatin 75 mg/m over 1 hour day 1 with darbepoetin 200 mug day 1 and pegfilgrastim 6 mg day 2 without/with BNP7787 before cisplatin was repeated every other week for up to 6 cycles. The primary end point was to differentiate between grade >/=2 neurotoxicity rates of 30% on [A] and 10% on [B]. Feasibility was prospectively defined as febrile neutropenia in <10% of patients and /=2 occurred in 32% on [A] and 29% on [B]. The incidence of febrile neutropenia was 4% on [A] and 3% on [B]. Treatment delays occurred in 13% and 20% of patients on [A] and [B], respectively. Completion rates for 3/6 cycles were 84%/51% on [A] and 84%/53% on [B]. Objective response rates were 55% on [A] and 51% on [B]. Median progression-free/overall survival times were 5.5/10.7 on [A] and 6.5/14.1 month on [B].This dose-dense treatment regimen is active, feasible, and tolerable. Its further investigation in the curative setting in non-small cell lung cancer should be considered. BNP7787 did not result in significant protection from neurotoxicity.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
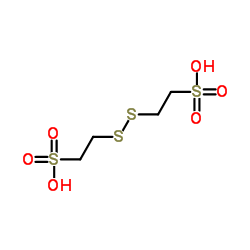 |
Coenzyme M
CAS:45127-11-5 |
C4H10O6S4 |
|
Mechanistic study of BNP7787-mediated cisplatin nephroprotec...
2011-02-01 [Cancer Chemother. Pharmacol. 67(2) , 381-91, (2011)] |
|
New approaches to drug discovery and development: a mechanis...
2003-07-01 [Cancer Chemother. Pharmacol. 52 Suppl 1 , S3-15, (2003)] |
|
Mechanistic study of BNP7787-mediated cisplatin nephroprotec...
2010-04-01 [Cancer Chemother. Pharmacol. 65(5) , 941-51, (2010)] |
|
Vibrational spectroscopic studies of mesna and dimesna.
2003-06-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 59(8) , 1791-8, (2003)] |
|
Analysis of BNP7787 thiol-disulfide exchange reactions in ph...
2009-04-01 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 877(10) , 857-66, (2009)] |