Reversible inactivation of horse liver aldehyde dehydrogenase by 2-hydroxyethyl disulfide.
J E Brotherton, V W Rodwell
Index: Physiol. Chem. Phys. 12(6) , 483-95, (1980)
Full Text: HTML
Abstract
Incubation of horse liver aldehyde dehydrogenase (aldehyde:NAD oxidoreductase, EC 1.2.1.3) with 2-hydroxyethyl disulfide formed mixed-disulfides between protein sulfhydryl groups and beta-mercaptoethanol. Reduction of aldehyde dehydrogenase activity may be associated with formation of one, or at most two, mixed-disulfides per dehydrogenase subunit. Characteristically in the case of a mixed-disulfide, inactivation was was reversed by addition of thiols. Other disulfides also inactivated aldehyde dehydrogenase. The pseudo first-order rate constants for the forward and reverse reactions (aldehyde dehydrogenase + 2-hydroxyethyl disulfide in equilibrium or formed from modified aldehyde dehydrogenase + beta-mercaptoethanol) were 0.70 and 2 liter mole-1 sec-1, respectively. The equilibrium constant was approximately 0.4. After extended incubation under conditions expected to result in complete modification of aldehyde dehydrogenase, 30% of the initial catalytic activity remained. This suggests that 2-hydroxyethyl disulfide-treated aldehyde dehydrogenase retains catalytic activity and that the sulfhydryl group modified by 2-hydroxyethyl disulfide is not essential for aldehyde dehydrogenase activity.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
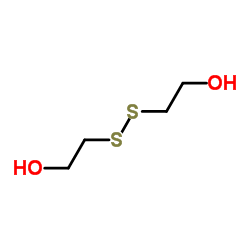 |
2,2'-DITHIODIETHANOL
CAS:1892-29-1 |
C4H10O2S2 |
|
Introgression of leginsulin, a cysteine-rich protein, and hi...
2015-03-25 [J. Agric. Food Chem. 63(11) , 2862-9, (2015)] |
|
Increasing proteome coverage for gel-based human tear proteo...
2015-07-01 [Biomed. Chromatogr. 29 , 1056-67, (2015)] |
|
Reduction processes in fast atom bombardment mass spectromet...
1990-10-01 [Biomed. Environ. Mass Spectrom. 19(10) , 628-34, (1990)] |
|
Purification and kinetic analysis of cytosolic and mitochond...
2015-02-01 [Exp. Parasitol. 149 , 65-73, (2015)] |
|
Hydroxyethyl disulfide as an efficient metabolic assay for c...
2012-06-01 [Toxicol. In Vitro 26(4) , 603-12, (2012)] |