4E-BP restrains eIF4E phosphorylation.
David Müller, Charline Lasfargues, Sally El Khawand, Amandine Alard, Robert J Schneider, Corinne Bousquet, Stéphane Pyronnet, Yvan Martineau
Index: Translation (Austin) 1 , e25819, (2016)
Full Text: HTML
Abstract
In eukaryotes, mRNA translation is dependent on the cap-binding protein eIF4E. Through its simultaneous interaction with the mRNA cap structure and with the ribosome-associated eIF4G adaptor protein, eIF4E physically posits the ribosome at the 5' extremity of capped mRNA. eIF4E activity is regulated by phosphorylation on a unique site by the eIF4G-associated kinase MNK. eIF4E assembly with the eIF4G-MNK sub-complex can be however antagonized by the hypophosphorylated forms of eIF4E-binding protein (4E-BP). We show here that eIF4E phosphorylation is dramatically affected by disruption of eIF4E-eIF4G interaction, independently of changes in MNK expression. eIF4E phosphorylation is actually strongly downregulated upon eIF4G shutdown or upon sequestration by hypophosphorylated 4E-BP, consequent to mTOR inhibition. Downregulation of 4E-BP renders eIF4E phosphorylation insensitive to mTOR inhibition. These data highlight the important role of 4E-BP in regulating eIF4E phosphorylation independently of changes in MNK expression.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
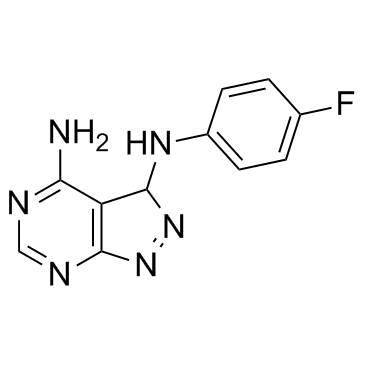 |
CGP 57380
CAS:522629-08-9 |
C11H9FN6 |
|
Targeting RNA transcription and translation in ovarian cance...
2014-09-15 [Oncotarget 5(17) , 7691-704, (2014)] |
|
MNKs act as a regulatory switch for eIF4E1 and eIF4E3 driven...
2014-01-01 [Nat. Commun. 5 , 5413, (2014)] |
|
Pharmacologic co-inhibition of Mnks and mTORC1 synergistical...
2015-02-28 [Cancer Lett. 357(2) , 612-23, (2015)] |
|
MNK1, a new MAP kinase-activated protein kinase, isolated by...
1997-04-15 [EMBO J. 16 , 1921-1933, (1997)] |
|
Negative regulation of protein translation by mitogen-activa...
2001-08-01 [Mol. Cell. Biol. 21 , 5500-5511, (2001)] |