General synthesis route to benanomicin-pradimicin antibiotics.
Minoru Tamiya, Ken Ohmori, Mitsuru Kitamura, Hirohisa Kato, Tadamasa Arai, Mami Oorui, Keisuke Suzuki
Index: Chemistry 13(35) , 9791-823, (2007)
Full Text: HTML
Abstract
A general approach to the regio- and stereoselective total synthesis of the benanomicin-pradimicin antibiotics (BpAs) is described. Construction of the aglycon has been achieved by 1) the diastereoselective ring-opening of a biaryl lactone by using (R)-valinol as a chiral nucleophile and 2) the stereocontrolled semi-pinacol cyclization of the aldehyde acetal by using SmI(2) in the presence of BF(3)OEt(2) and a proton source to afford the ABCD tetracyclic monoprotected diol. This strategy enabled us to control the two stereogenic sites in the B ring (C-5 and C-6) and the regioselective introduction of the carbohydrate moiety. The ABCD tetracycle could serve as an ideal platform for the divergent access to various BpAs. The amino acid (D-alanine) was introduced onto the ABCD tetracycle. Glycosylation was promoted by the combination of Cp(2)HfCl(2) and AgOTf (1:2 ratio). Construction of the E ring followed by deprotection completed the first total synthesis of benanomicin A (2 a), benanomicin B (2 b), and pradimicin A (1 a). The route is flexible enough to allow the synthesis of other congeners differing in their amino acid and carbohydrate moieties.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
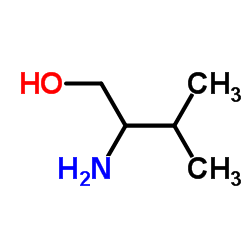 |
2-Amino-3-methylbutan-1-ol
CAS:16369-05-4 |
C5H13NO |
|
Chiral bis(amino alcohol)oxalamide gelators-gelation propert...
2003-11-21 [Chemistry 9(22) , 5567-80, (2003)] |
|
Synthesis of highly enantioenriched chiral alpha-aminoorgano...
2009-08-21 [J. Org. Chem. 74(16) , 5822-38, (2009)] |
|
Asymmetric synthesis of 8-aminoindolizidine from chiral 2-py...
2008-11-07 [J. Org. Chem. 73(21) , 8376-81, (2008)] |
|
Order of binding of substrate to valyl-tRNA synthetase from ...
1987-04-01 [Biochem. Int. 14(4) , 597-603, (1987)] |
|
Dietary low-dose sucrose modulation of IQ-induced genotoxici...
2003-06-19 [Mutat. Res. 527(1-2) , 91-7, (2003)] |