Asymmetric synthesis of 8-aminoindolizidine from chiral 2-pyrroleimines.
Vincenzo Giulio Albano, Andrea Gualandi, Magda Monari, Diego Savoia
Index: J. Org. Chem. 73(21) , 8376-81, (2008)
Full Text: HTML
Abstract
1-Allyl-2-pyrroleimines obtained from (S)-valinol and (S)-phenylglycinol underwent highly diastereoselective addition of allylmagnesium chloride, used in excess amounts, to give the corresponding secondary amines with concomitant allyl to (Z)-1-propenyl isomerization of the 1-pyrrole substituent. Transformation of the 2-amino alcohol moiety to an oxazolidinone, or its cleavage and subsequent N-protection, followed by ring-closing metathesis of the two alkene groups gave the unsaturated bicyclic compound. Full hydrogenation of the alkene function and the aromatic rings afforded the indolizidine derivative as a mixture of two or three diastereomers with a ratio which was dependent on the nature of both the N-substituent and the catalyst. The two prevalent diastereomers were isolated, and their configuration was determined by X-ray crystallographic analysis.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
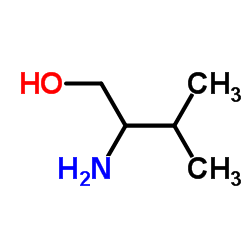 |
2-Amino-3-methylbutan-1-ol
CAS:16369-05-4 |
C5H13NO |
|
Chiral bis(amino alcohol)oxalamide gelators-gelation propert...
2003-11-21 [Chemistry 9(22) , 5567-80, (2003)] |
|
Synthesis of highly enantioenriched chiral alpha-aminoorgano...
2009-08-21 [J. Org. Chem. 74(16) , 5822-38, (2009)] |
|
General synthesis route to benanomicin-pradimicin antibiotic...
2007-01-01 [Chemistry 13(35) , 9791-823, (2007)] |
|
Order of binding of substrate to valyl-tRNA synthetase from ...
1987-04-01 [Biochem. Int. 14(4) , 597-603, (1987)] |
|
Dietary low-dose sucrose modulation of IQ-induced genotoxici...
2003-06-19 [Mutat. Res. 527(1-2) , 91-7, (2003)] |