Meprin A and meprin alpha generate biologically functional IL-1beta from pro-IL-1beta.
Christian Herzog, Randy S Haun, Varsha Kaushal, Philip R Mayeux, Sudhir V Shah, Gur P Kaushal, Christian Herzog, Randy S Haun, Varsha Kaushal, Philip R Mayeux, Sudhir V Shah, Gur P Kaushal
Index: Biochem. Biophys. Res. Commun. 379(4) , 904-8, (2009)
Full Text: HTML
Abstract
The present study demonstrates that both oligomeric metalloendopeptidase meprin A purified from kidney cortex and recombinant meprin alpha are capable of generating biologically active IL-1beta from its precursor pro-IL-1beta. Amino-acid sequencing analysis reveals that meprin A and meprin alpha cleave pro-IL-1beta at the His(115)-Asp(116) bond, which is one amino acid N-terminal to the caspase-1 cleavage site and five amino acids C-terminal to the meprin beta site. The biological activity of the pro-IL-1beta cleaved product produced by meprin A, determined by proliferative response of helper T-cells, was 3-fold higher to that of the IL-1beta product produced by meprin beta or caspase-1. In a mouse model of sepsis induced by cecal ligation puncture that results in elevated levels of serum IL-1beta, meprin inhibitor actinonin significantly reduces levels of serum IL-1beta. Meprin A and meprin alpha may therefore play a critical role in the production of active IL-1beta during inflammation and tissue injury.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
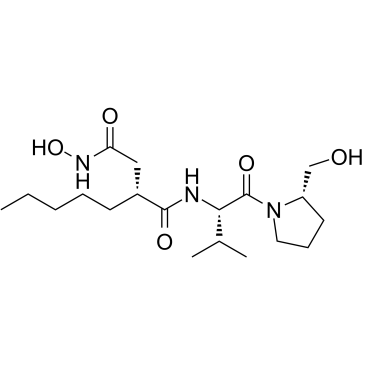 |
ACTINONIN
CAS:13434-13-4 |
C19H35N3O5 |
|
Proteome-wide analysis of the amino terminal status of Esche...
2015-07-01 [Proteomics 15 , 2503-18, (2015)] |
|
Quality control of mitochondrial protein synthesis is requir...
2015-10-26 [J. Cell Biol. 211 , 373-89, (2015)] |
|
Concanavalin-A-induced autophagy biomarkers requires membran...
2012-09-01 [Glycobiology 22(9) , 1245-55, (2012)] |
|
A role for metalloendopeptidases in the breakdown of the gut...
2011-12-01 [Endocrinology 152 , 4630-40, (2011)] |
|
The novel endomorphin degradation blockers Tyr-Pro-DClPhe-Ph...
2010-07-01 [Chem. Biol. Drug Des. 76(1) , 77-81, (2010)] |