ACTINONIN
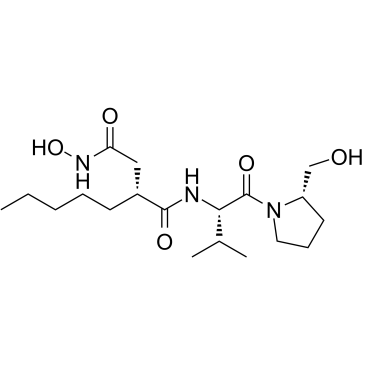
ACTINONIN structure
|
Common Name | ACTINONIN | ||
|---|---|---|---|---|
| CAS Number | 13434-13-4 | Molecular Weight | 385.498 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C19H35N3O5 | Melting Point | 137-139ºC | |
| MSDS | USA | Flash Point | N/A | |
|
Proteome-wide analysis of the amino terminal status of Escherichia coli proteins at the steady-state and upon deformylation inhibition.
Proteomics 15 , 2503-18, (2015) A proteome wide analysis was performed in Escherichia coli to identify the impact on protein N-termini of actinonin, an antibiotic specifically inhibiting peptide deformylase (PDF). A strategy and tool suite (SILProNaQ) was employed to provide large-scale qua... |
|
|
Quality control of mitochondrial protein synthesis is required for membrane integrity and cell fitness.
J. Cell Biol. 211 , 373-89, (2015) Mitochondrial ribosomes synthesize a subset of hydrophobic proteins required for assembly of the oxidative phosphorylation complexes. This process requires temporal and spatial coordination and regulation, so quality control of mitochondrial protein synthesis... |
|
|
Concanavalin-A-induced autophagy biomarkers requires membrane type-1 matrix metalloproteinase intracellular signaling in glioblastoma cells.
Glycobiology 22(9) , 1245-55, (2012) Pre-clinical trials for cancer therapeutics support the anti-neoplastic properties of the lectin from Canavalia ensiformis (Concanavalin-A, ConA) in targeting apoptosis and autophagy in a variety of cancer cells. Given that membrane type-1 matrix metalloprote... |
|
|
A role for metalloendopeptidases in the breakdown of the gut hormone, PYY 3-36.
Endocrinology 152 , 4630-40, (2011) Peptide YY(3-36) (PYY(3-36)) is a gut hormone that acts on Y2 receptors to reduce appetite. Obese humans are sensitive to the anorectic effects of PYY(3-36) and display a blunted postprandial rise in PYY(3-36). Bariatric surgery results in increased circulati... |
|
|
The novel endomorphin degradation blockers Tyr-Pro-DClPhe-Phe-NH (EMDB-1) and Tyr-Pro-Ala-NH (EMDB-2) prolong endomorphin-2 action in rat ileum in vitro.
Chem. Biol. Drug Des. 76(1) , 77-81, (2010) The endogenous opioid system is involved in the control of gastrointestinal (GI) motility. The potential use of endogenous MOR ligands, endomorphins (EMs), as therapeutics is limited because of their rapid enzymatic degradation and short duration of action. T... |
|
|
Meprin A and meprin alpha generate biologically functional IL-1beta from pro-IL-1beta.
Biochem. Biophys. Res. Commun. 379(4) , 904-8, (2009) The present study demonstrates that both oligomeric metalloendopeptidase meprin A purified from kidney cortex and recombinant meprin alpha are capable of generating biologically active IL-1beta from its precursor pro-IL-1beta. Amino-acid sequencing analysis r... |
|
|
In vitro and ex vivo activity of peptide deformylase inhibitors against Mycobacterium tuberculosis H37Rv.
Int. J. Antimicrob. Agents 34(3) , 226-30, (2009) Bacterial peptide deformylase (PDF) catalyses removal of the N-terminal formyl group of proteins and is essential for protein maturation, growth and survival of bacteria. Thus, PDF appears to be a good antimycobacterial drug target. In the present study, vari... |
|
|
News from an ancient world: two novel astacin metalloproteases from the horseshoe crab.
J. Mol. Biol. 385(1) , 236-48, (2009) In this work, we report the cloning, heterologous expression, and characterization of two novel astacin proteases from the chelicerate Limulus polyphemus (horseshoe crab), designated as LAST (Limulus astacin) and LAST_MAM (Limulus astacin containing a MAM dom... |
|
|
Mutations in three distinct loci cause resistance to peptide deformylase inhibitors in Bacillus subtilis.
Antimicrob. Agents Chemother. 53(4) , 1673-8, (2009) Bacillus subtilis mutants with resistance against peptide deformylase inhibitors were isolated. All showed a bypass of the pathway through mutations in three genes required for formylation of Met-tRNA(fMet), fmt, folD, and glyA. glyA corresponds to a yet unch... |
|
|
A meprin inhibitor suppresses atherosclerotic plaque formation in ApoE-/- mice.
Atherosclerosis 207(1) , 84-92, (2009) Meprin is a member of the astacin family of zinc metalloendopeptidases. It is widely distributed in the body, and hydrolyzes and inactivates several endogenous vasoactive peptides, some of which could alter various functions of cells in the arterial wall. We ... |