Journal of the American Society for Mass Spectrometry
2013-06-01
Gas-phase fragmentation of oligoproline peptide ions lacking easily mobilizable protons.
Magdalena Rudowska, Robert Wieczorek, Alicja Kluczyk, Piotr Stefanowicz, Zbigniew Szewczuk
Index: J. Am. Soc. Mass Spectrom. 24 , 846-56, (2013)
Full Text: HTML
Abstract
The fragmentation of peptides containing quaternary ammonium group, but lacking easily mobilizable protons, was examined with the aid of deuterium-labeled analogs and quantum-chemical modeling. The fragmentation of oligoproline containing quaternary ammonium group involves the mobilization of hydrogens localized at α- and γ- or δ-carbon atoms in the pyrrolidine ring of proline. The study of the dissociation pattern highlights the unusual proline residue behavior during MS/MS experiments of peptides.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
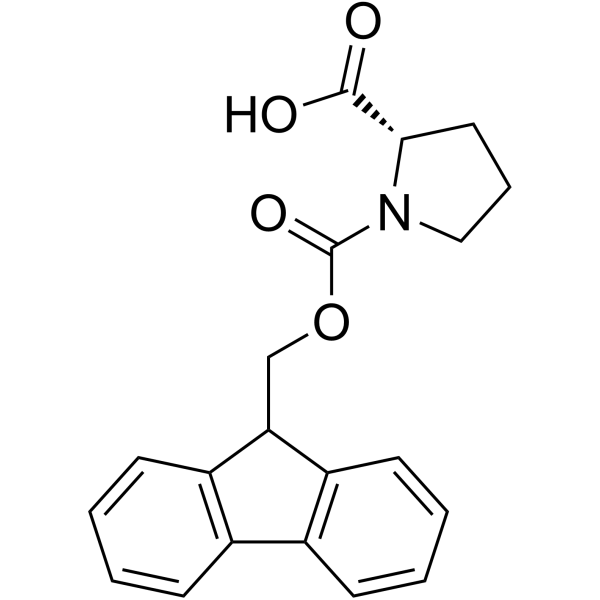 |
Fmoc-Pro-OH
CAS:71989-31-6 |
C20H19NO4 |
Related Articles:
More...
|
Metabolomic serum profiling detects early-stage high-grade s...
2015-02-06 [J. Proteome Res. 14(2) , 917-27, (2015)] |
|
Non-covalent photo-patterning of gelatin matrices using cage...
2015-01-01 [Macromol. Biosci. 15(1) , 52-62, (2015)] |
|
Small molecule inhibitors of the neuropilin-1 vascular endot...
2010-03-11 [J. Med. Chem. 53 , 2215-26, (2010)] |
|
Cell-penetrating apoptotic peptide/p53 DNA nanocomplex as ad...
2014-10-06 [Mol. Pharm. 11(10) , 3352-60, (2014)] |
|
Complete ON/OFF photoswitching of the motility of a nanobiom...
2014-05-27 [ACS Nano 8(5) , 4157-65, (2014)] |