Change in reaction pathway in the reduction of 3,5-di-tert-butyl-1,2-benzoquinone with increasing concentrations of 2,2,2-trifluoroethanol.
Norma A Macías-Ruvalcaba, Noriko Okumura, Dennis H Evans
Index: J. Phys. Chem. B 110(43) , 22043-7, (2006)
Full Text: HTML
Abstract
The electrochemical reduction of 3,5-di-tert-butyl-1,2-benzoquinone, 1, has been studied in acetonitrile with added 2,2,2-trifluoroethanol, 2. At low concentrations of 2 the reaction proceeds by the following pathway: reduction of the quinone (Q) to its anion radical (Q*-) followed by complexation of the anion radical with 2 (HA) and the further reduction of the hydrogen-bonded complex (Q*- (HA)) to form HQ- and A-. The latter reaction is a concerted proton and electron- transfer reaction (CPET). At higher concentrations of 2, the pathway changes. The first steps remain the same, but now Q*- (HA) is reduced to HQ- via a disproportionation reaction with Q*- along with proton transfer from HA to Q*- to form HQ* which is reduced to HQ-. The only mechanism that could be found which would account for all of the data involves proton transfer to Q*- occurring within a higher complex, Q*-(HA)3.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
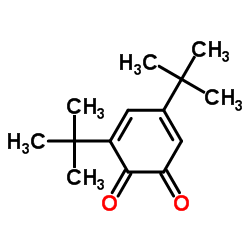 |
3,5-DI-TERT-BUTYL-O-BENZOQUINONE
CAS:3383-21-9 |
C14H20O2 |
|
Reactions of the phosphinidene-bridged complexes [Fe2(η5-C5H...
2012-12-28 [Dalton Trans. 41(48) , 14498-513, (2012)] |
|
Versatile chemical transformations of benzoxazole based liga...
2012-02-20 [Inorg. Chem. 51(4) , 2588-96, (2012)] |
|
Determination of natural thiols by liquid chromatography aft...
[J. Chromatogr. A. 420(2) , 404-10, (1987)] |
|
Stability of colistimethate sodium in aqueous solution.
2012-12-01 [J. Inorg. Biochem. 103(3) , 389-95, (2009)] |
|
A dicopper complex with distant metal centers. Structure, ma...
2008-01-01 [J. Inorg. Biochem. 102(5-6) , 1227-35, (2008)] |