A disposable voltammetric cell for determining the titratable acidity in vinegar.
Akira Kotani, Yuji Miyaguchi, Dai Harada, Fumiyo Kusu
Index: Anal. Sci. 19(11) , 1473-6, (2003)
Full Text: HTML
Abstract
A disposable voltammetric cell using three pencil leads as working, reference, and counter electrodes was developed for determining the titratable acidity, i.e. the acid content in vinegar. The materials of the pencil leads were graphite-reinforcement carbons (GRCs). A voltammetric determination of acid was made by measuring the reduction prepeak current of 3,5-di-t-butyl-1,2-benzoquinone (DBBQ) due to the presence of acids in unbuffered solution. The potential stability of the pseudo-reference electrode of GRC was examined. The prepeak current was found to be proportional to the acetic acid concentration from 0.05 to 2.7 mM with a correlation coefficient of 0.999. The cell-to-cell reproducibility for 1 mM acetic acid was evaluated with ten individual disposable cells. The RSD of the prepeak current and the SD of the prepeak potential were 2.56% and 0.008, respectively. The titratable acidity in five vinegar samples was determined by voltammetry using disposable cells and compared with that of the titratable acidity determined by the conventional potentiometric titration method. We then observed the results by both methods, and found a correlation coefficient of 0.972. As such, the voltammetry using disposable-cell required only one thousandth the volume of a vinegar sample for the titration method. The disposable cell was superior to the conventional electrochemical cell, in terms of facility, environment-friendly, and economy, and thus a sensor using the present cell would be useful for routine work in the quality control of vinegar.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
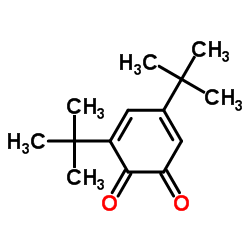 |
3,5-DI-TERT-BUTYL-O-BENZOQUINONE
CAS:3383-21-9 |
C14H20O2 |
|
Reactions of the phosphinidene-bridged complexes [Fe2(η5-C5H...
2012-12-28 [Dalton Trans. 41(48) , 14498-513, (2012)] |
|
Versatile chemical transformations of benzoxazole based liga...
2012-02-20 [Inorg. Chem. 51(4) , 2588-96, (2012)] |
|
Determination of natural thiols by liquid chromatography aft...
[J. Chromatogr. A. 420(2) , 404-10, (1987)] |
|
Stability of colistimethate sodium in aqueous solution.
2012-12-01 [J. Inorg. Biochem. 103(3) , 389-95, (2009)] |
|
A dicopper complex with distant metal centers. Structure, ma...
2008-01-01 [J. Inorg. Biochem. 102(5-6) , 1227-35, (2008)] |