Nucleosides, Nucleotides & Nucleic Acids
2009-05-01
The "Corey's reagent," 3,5-di-tert-butyl-1,2-benzoquinone, as a modifying agent in the synthesis of fluorescent and double-headed nucleosides.
Victor A Timoshchuk, Richard I Hogrefe
Index: Nucleosides Nucleotides Nucleic Acids 28(5) , 464-72, (2009)
Full Text: HTML
Abstract
A new method for the synthesis of fluorescent nucleosides has been developed. It has been shown that a reaction of benzoquinone with aminopropenyl group at C-5-position of 2'-deoxyuridine or 2'-deoxycytidine and aminopropynyl group at the C-7-position of 8-aza-7-deazaadenosine under extremely mild conditions affords conjugated benzoxazole derivatives of nucleosides, which possess strong fluorescent properties. In a similar reaction 5'-amino-5'-deoxy-nucleosides form double-headed nucleoside derivatives with benzoxazole attached at C-4'-position.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
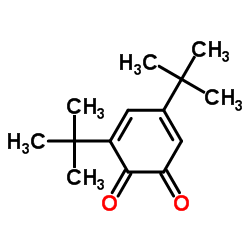 |
3,5-DI-TERT-BUTYL-O-BENZOQUINONE
CAS:3383-21-9 |
C14H20O2 |
Related Articles:
More...
|
Reactions of the phosphinidene-bridged complexes [Fe2(η5-C5H...
2012-12-28 [Dalton Trans. 41(48) , 14498-513, (2012)] |
|
Versatile chemical transformations of benzoxazole based liga...
2012-02-20 [Inorg. Chem. 51(4) , 2588-96, (2012)] |
|
Determination of natural thiols by liquid chromatography aft...
[J. Chromatogr. A. 420(2) , 404-10, (1987)] |
|
Stability of colistimethate sodium in aqueous solution.
2012-12-01 [J. Inorg. Biochem. 103(3) , 389-95, (2009)] |
|
A dicopper complex with distant metal centers. Structure, ma...
2008-01-01 [J. Inorg. Biochem. 102(5-6) , 1227-35, (2008)] |