Synthesis and CD structural studies of CD52 peptides and glycopeptides.
Benjamin M Swarts, Yu-Cheng Chang, Honggang Hu, Zhongwu Guo
Index: Carbohydr. Res. 343 , 2894-2902, (2008)
Full Text: HTML
Abstract
The syntheses of five natural and N-terminal acetylated peptides and glycopeptides of the CD52 antigen are described. Solid phase peptide synthesis was employed in the construction of the target compounds from Fmoc-protected commercial amino acids and synthetic glycan-asparagine conjugates. Circular dichroism studies of the synthetic targets showed that they exist as random coils in solution, and no significant change in secondary structure was observed when the CD52 peptide was either acetylated at the N-terminus or glycosylated at the Asn(3) residue with a disaccharide or a fucose-containing branched trisaccharide.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
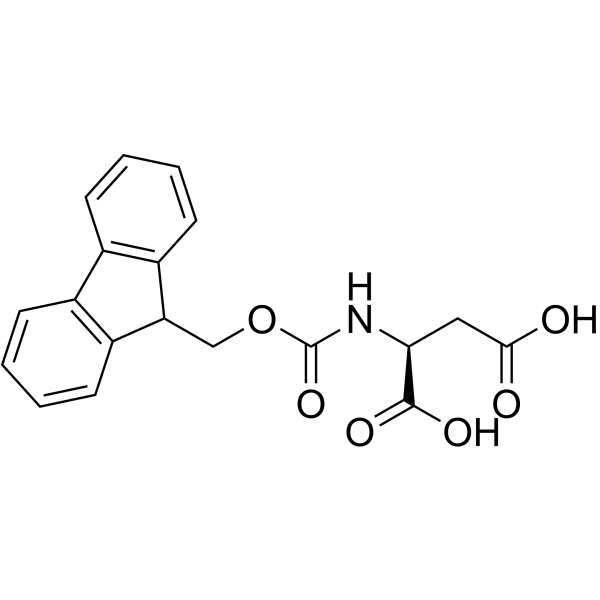 |
Fmoc-Asp-OH
CAS:119062-05-4 |
C19H17NO6 |
|
Synthesis of N-linked glycopeptides via solid-phase aspartyl...
2010-08-21 [Org. Biomol. Chem. 8 , 3723-3733, (2010)] |
|
The aspartimide problem in Fmoc-based SPPS. Part I.
2003-01-01 [J. Pept. Sci. 9 , 36-46, (2003)] |
|
The aspartimide problem in Fmoc-based SPPS. Part II.
2003-08-01 [J. Pept. Sci. 9 , 518-526, (2003)] |
|
The aspartimide problem in Fmoc-based SPPS. Part III.
2005-10-01 [J. Pept. Sci. 11 , 650-657, (2005)] |
|
On-resin convergent synthesis of a glycopeptide from HIV gp1...
2011-01-01 [Methods Mol. Biol. 751 , 343-355, (2011)] |