Fmoc-Asp-OH
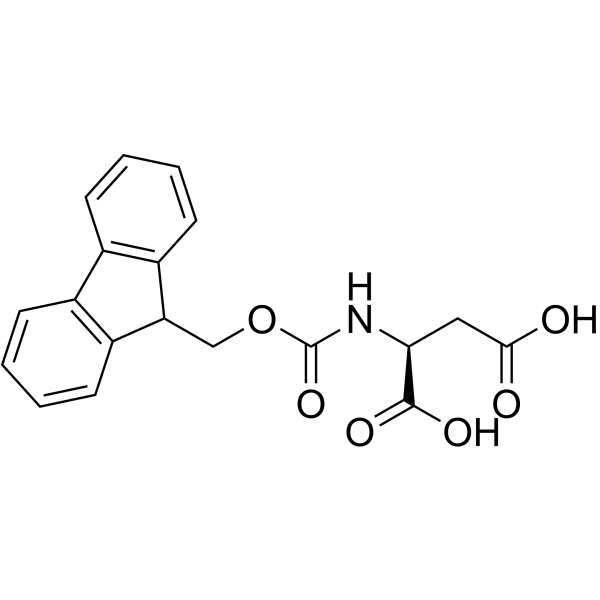
Fmoc-Asp-OH structure
|
Common Name | Fmoc-Asp-OH | ||
|---|---|---|---|---|
| CAS Number | 119062-05-4 | Molecular Weight | 355.341 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 587.2±45.0 °C at 760 mmHg | |
| Molecular Formula | C19H17NO6 | Melting Point | N/A | |
| MSDS | USA | Flash Point | 308.9±28.7 °C | |
Use of Fmoc-Asp-OH(((9H-Fluoren-9-yl)methoxy)carbonyl)-L-aspartic acid is an aspartic acid derivative[1]. |
| Name | N-Fmoc-L-Asparagine |
|---|---|
| Synonym | More Synonyms |
| Description | (((9H-Fluoren-9-yl)methoxy)carbonyl)-L-aspartic acid is an aspartic acid derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 587.2±45.0 °C at 760 mmHg |
| Molecular Formula | C19H17NO6 |
| Molecular Weight | 355.341 |
| Flash Point | 308.9±28.7 °C |
| Exact Mass | 355.105591 |
| PSA | 112.93000 |
| LogP | 3.55 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.628 |
| InChIKey | KSDTXRUIZMTBNV-INIZCTEOSA-N |
| SMILES | O=C(O)CC(NC(=O)OCC1c2ccccc2-c2ccccc21)C(=O)O |
| Storage condition | −20°C |
| Hazard Codes | Xn |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
|
Synthesis of N-linked glycopeptides via solid-phase aspartylation.
Org. Biomol. Chem. 8 , 3723-3733, (2010) An efficient strategy for the preparation of N-linked glycopeptides is described. The method relies on the use of side chain protecting groups on aspartic acid residues, namely the allyl and Dmab este... |
|
|
The aspartimide problem in Fmoc-based SPPS. Part I.
J. Pept. Sci. 9 , 36-46, (2003) A variety of Asp beta-carboxy protecting groups, Hmb backbone protection and a range of Fmoc cleavage protocols have been employed in syntheses of the model hexapeptide H-VKDGYI-OH to investigate the ... |
|
|
The aspartimide problem in Fmoc-based SPPS. Part II.
J. Pept. Sci. 9 , 518-526, (2003) The sequence dependence of base-catalysed aspartmide formation during Fmoc-based SPPS was systematically studied employing the peptide models H-Val-Lys-Asp-Xaa-Tyr-Ile-OH. The extent of formation of a... |
| Fmoc-Asp-OH |
| L-Aspartic acid, N-[(9H-fluoren-9-ylmethoxy)carbonyl]- |
| N-[(9H-Fluoren-9-ylmethoxy)carbonyl]-L-aspartic acid |
| N-Fmoc-L-aspartic acid |
| MFCD00237654 |
| (2S)-2-{[(9H-Fluoren-9-ylmethoxy)carbonyl]amino}succinic acid |
| Fmoc-L-aspartic acid |

