| Structure | Name/CAS No. | Articles |
|---|---|---|
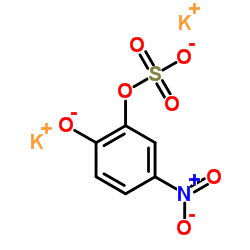 |
Dipotassium 5-nitro-2-oxidophenyl sulfate
CAS:14528-64-4 |
A A Farooqui, A N Yusufi
Index: J. Neurochem. 27(5) , 1191-5, (1976)
Full Text: HTML
(1) Arylsulphatase of the silkworm Bombyx mori was partially purified using ammonium sulphate fractionation, ethanol precipitation, Sephadex G-200 gel filtration and Con-A Sepharose chromatography. (2) The purified enzyme preparation was not homogeneous but showed no beta-glucuronidase or beta-galactosidase activities. (3) The kinetic properties of the enzyme indicated that it could be classified under type-2 arylsulphatases of vertebrates. (4) The purified enzyme shows very little activity towards p-nitrophenyl sulphate and none towards cerebroside 3-sulphate.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
Dipotassium 5-nitro-2-oxidophenyl sulfate
CAS:14528-64-4 |
C6H3K2NO7S |
|
Arylsulfatase A bound to poly(butyl cyanoacrylate) nanoparti...
2014-07-01 [Pharmazie 69(7) , 518-24, (2014)] |
|
Investigation of catalytic loop structure, dynamics, and fun...
2012-05-31 [J. Phys. Chem. B 116(21) , 6166-76, (2012)] |
|
Isolation and characterization of rat hepatic ascorbic acid-...
1987-01-01 [Enzyme 37(3) , 134-40, (1987)] |
|
Chemical characterization and substrate specificity of rabbi...
1980-07-10 [Biochim. Biophys. Acta 614(1) , 92-101, (1980)] |
|
A direct-colouring, metal precipitation method for the demon...
1984-05-01 [Histochem. J. 16(5) , 501-6, (1984)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved