| Structure | Name/CAS No. | Articles |
|---|---|---|
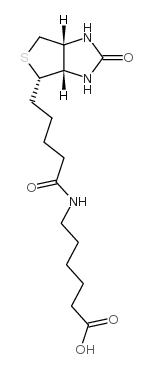 |
N-Biotinyl-6-aminohexanoic acid
CAS:72040-64-3 |
Xiaoxiong Zeng, Yi Sun, Hong Ye, Jun Liu, Xiaoli Xiang, Bei Zhou, Hirotaka Uzawa
Index: Carbohydr. Res. 342 , 1244-1248, (2007)
Full Text: HTML
A convenient chemoenzymatic procedure for the synthesis of p-aminophenyl glycosides of sialyl N-acetyllactosaminide has been developed from p-nitrophenyl N-acetyl-beta-D-glucosaminide as starting material through three steps: synthesis of p-nitrophenyl N-acetyllactosaminide with beta-D-galactosidase, chemical reduction of the p-nitrophenyl group, and sialylation with sialyltransferase. The p-aminophenyl glycosides were then successfully biotin-labeled through the coupling with N-(+)-biotinyl-6-aminohexanoic acid to afford biotinylated oligosaccharides with an aminohexanosyl group and phenyl group as the spacers between the biotin and glycan. Furthermore, the biotin-labeled sugars were shown to be useful for immobilization and assay of the carbohydrate-lectin interactions by an optical biosensor based on surface plasmon resonance.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
N-Biotinyl-6-aminohexanoic acid
CAS:72040-64-3 |
C16H27N3O4S |
|
In vivo labeling of Escherichia coli cell envelope pr...
[Appl. Environ. Microbiol. 59 , 663-668, (2993)] |
|
(-)-7'-Isothiocyanato-11-hydroxy-1',1'-dimethylheptylhexahyd...
2005-12-01 [Mol. Pharmacol. 68 , 1623-35, (2005)] |
|
microAg particle-based molecular sensing/recognition via sur...
2007-01-15 [Biosens. Bioelectron. 22 , 1000-1005, (2007)] |
|
Survival of baboon biotin-X-N-hydroxysuccinimide and (111)In...
2005-02-01 [Vox Sang. 88 , 122-129, (2005)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved