| Structure | Name/CAS No. | Articles |
|---|---|---|
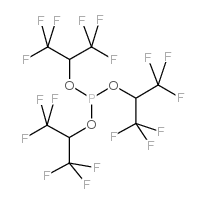 |
tris(1,1,1,3,3,3-hexafluoro-2-propyl) phosphite
CAS:66470-81-3 |
| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
tris(1,1,1,3,3,3-hexafluoro-2-propyl) phosphite
CAS:66470-81-3 |