Efficacy and safety of ethynodiol diacetate, 1 mg, with ethinyl estradiol, 35 micrograms, with an emphasis on contraceptive efficacy. A phase IV trial.
A J Friedman, J Wheeler
Index: J. Reprod. Med. 36(4 Suppl) , 328-33, (1991)
Full Text: HTML
Abstract
A phase IV trial evaluated the efficacy and safety of a monophasic oral contraceptive formulation, ethynodiol diacetate, 1 mg, plus ethinyl estradiol, 35 micrograms (EDA 1 mg with EE 35 micrograms) (Demulen 1/35). Nine hundred eighty-three community-based obstetrician-gynecologists treated a total of 7,759 patients with EDA 1 mg with EE 35 micrograms for one to eight months. Clinical evaluation forms on 6,382 patients were amenable to analysis for safety (including breakthrough bleeding, ovarian cyst formation and complexion changes); 5,412 patients were evaluable for efficacy (prevention of pregnancy), with a total of 21,440 cycles recorded. The study results were interpreted in terms of the impact on clinical management of oral contraceptive users and the methods, strengths and weaknesses of phase IV trials, particularly as they relate to confirmation of the results reported here.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
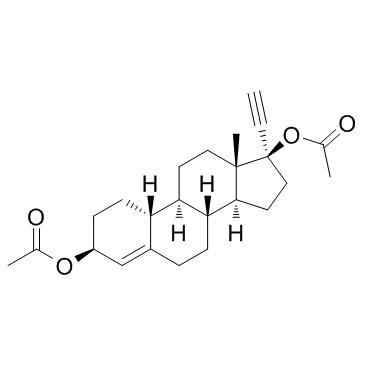 |
Ethynodiol diacetate
CAS:297-76-7 |
C24H32O4 |
|
Suppressive effect of neonatal treatment with a phytoestroge...
2005-01-15 [Brain Res. Bull. 64(5) , 449-54, (2005)] |
|
New observations on endometrial physiology after transcervic...
2004-12-01 [Fertil. Steril. 82(6) , 1700-4, (2004)] |
|
Membrane-initiated steroid signaling (MISS): genomic steroid...
2003-05-01 [J. Steroid Biochem. Mol. Biol. 85(1) , 9-23, (2003)] |
|
Do AshSplit haemodialysis catheters provide better flow rate...
2007-01-01 [Ren. Fail. 29(6) , 721-9, (2007)] |
|
Incidence of ovarian cyst formation in women taking ethynodi...
1991-04-01 [J. Reprod. Med. 36(4 Suppl) , 345-9, (1991)] |