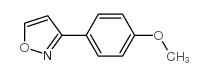
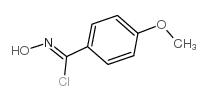
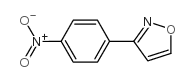
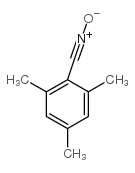
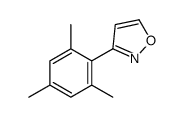
|
~% |
|
~% |
|
~% |
|
~0%
Detail
|
|
~0%
Detail
|
|
~0%
Detail
|
|
~0%
Detail
|
|
~0%
Detail
|
|
~0%
Detail
|
|
~0%
Detail
|
|
~90%
Detail
|
|
~92%
Detail
|
|
~50%
Detail
|
|
~12%
Detail
|
|
~87%
Detail
|
|
~82%
Detail
|
|
~87%
Detail
|
|
~88%
Detail
|
|
~25%
Detail
|
|
~83%
Detail
|
|
~22%
Detail
|
|
~16%
Detail
|
|
~% |
|
~13%
Detail
|
|
~10%
Detail
|
|
~75%
Detail
|
|
~18%
Detail
|
|
~79%
Detail
|
|
~83%
Detail
|
|
~44%
Detail
|
|
~94%
Detail
|
|
~96%
Detail
|
|
~60% |
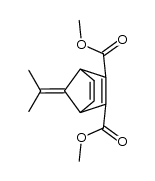
![dimethyl bicyclo[2.2.1]hepta-2,5-diene-5,6-dicarboxylate Structure](https://image.chemsrc.com/caspic/423/947-57-9.png)
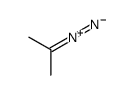
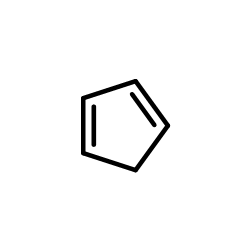
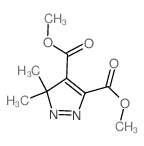
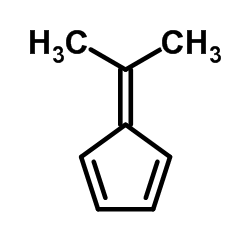
![Dimethyl 7-Oxabicyclo[2.2.1]hepta-2,5-diene-2,3-dicarboxylate Structure](https://image.chemsrc.com/caspic/115/1829-60-3.png)




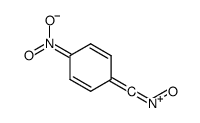
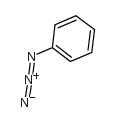

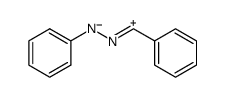


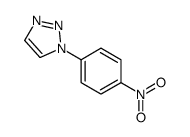
![1-(4-nitro-phenyl)-1H-[1,2,3]triazole-4,5-dicarboxylic acid dimethyl ester Structure](https://image.chemsrc.com/caspic/046/4999-94-4.png)