| Structure | Name/CAS No. | Articles |
|---|---|---|
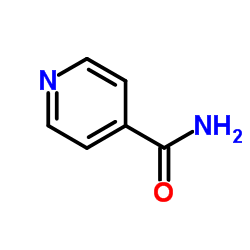 |
Isonicotinamide
CAS:1453-82-3 |
Gareth Arnott, Heloise Brice, Jonathan Clayden, Emma Blaney
Index: Org. Lett. 10(14) , 3089-92, (2008)
Full Text: HTML
Treatment of N-arylisonicotinamides with trifluoromethanesulfonic anhydride triggers intramolecular nucleophilic attack of the aryl ring on the 4-position of the pyridinium intermediate. The products are spirocyclic dihydropyridines which can be converted to valuable spirocyclic piperidines related to biologically active molecules such as MK-677.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
Isonicotinamide
CAS:1453-82-3 |
C6H6N2O |
|
Targeting SRPK1 to control VEGF-mediated tumour angiogenesis...
2014-07-29 [Br. J. Cancer 111(3) , 477-85, (2014)] |
|
[Fe(CN)5(isoniazid)](3-): an iron isoniazid complex with red...
2014-11-01 [J. Inorg. Biochem. 140 , 236-44, (2014)] |
|
Measuring induction times and crystal nucleation rates.
2015-01-01 [Faraday Discuss. 179 , 199-214, (2015)] |
|
A novel strategy for pharmaceutical cocrystal generation wit...
2015-01-01 [Pharm. Res. 32(1) , 47-60, (2015)] |
|
Zero-, one- and two-dimensional hydrogen-bonded structures i...
2009-03-01 [Acta Crystallogr. C 65(Pt 3) , o103-7, (2009)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2024 ChemSrc All Rights Reserved