Catalytic decomposition of the reactive dye Uniblue A on hematite. Modeling of the reactive surface.
F Herrera, A Lopez, G Mascolo, P Albers, J Kiwi
Index: Water Res. 35(3) , 750-60, (2001)
Full Text: HTML
Abstract
Experimental results from the adsorption and subsequent catalytic combustion of the reactive dye Uniblue A on hematite indicate that this iron oxide can be used as an affordable catalyst for environmental purposes. Uniblue A was adsorbed on hematite and the products of the catalytic oxidation in O2 atmosphere were analyzed by thermal programmed gas-chromatography/mass spectrometry (STDS-GC-MS) analysis. The catalytic combustion of Uniblue A in the presence of hematite led to about 40% conversion of the dye C-content into CO2 at T = 275 degrees C. The activation energy (Ea) for the desorption of CO2 and other polyaromatic hydrocarbons (PAHs) from the hematite surface was determined to be 23.4 kcal mol-1. Identification of the species of Uniblue A in solution and those existing on the hematite surface was carried out in the framework of the generalized two-layer diffuse model. The modeling of the amount of dye absorbed on hematite is in good agreement with the experimental data.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
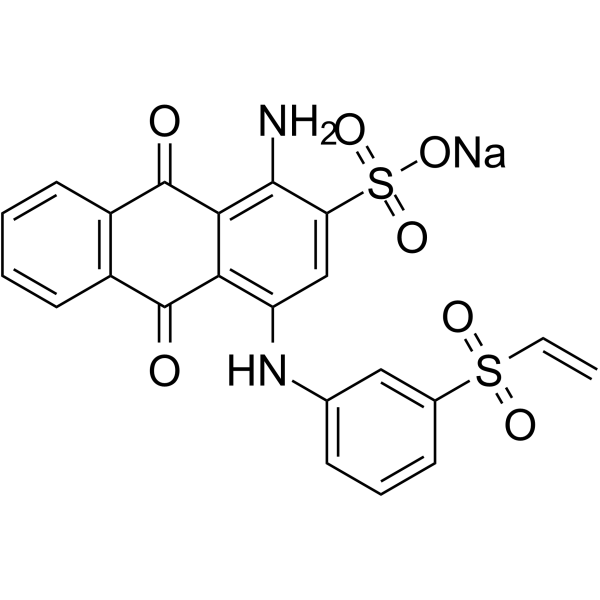 |
Uniblue A sodium salt
CAS:14541-90-3 |
C22H15N2NaO7S2 |
|
Lysine-directed staining of proteins for MS-based analyses.
2013-02-01 [Electrophoresis 34(3) , 401-4, (2013)] |
|
Interaction of anthraquinone dyes with lysozyme: evidences f...
2010-03-15 [J. Hazard. Mater. 175(1-3) , 985-91, (2010)] |
|
Discrimination between UTP- and P2-purinoceptor-mediated dep...
2012-03-05 [Br. J. Pharmacol. 115(3) , 427-32, (1995)] |
|
Accelerated identification of proteins by mass spectrometry ...
2012-01-01 [PLoS ONE 7(2) , e31438, (2012)] |
|
Concomitant blockade of P2X-receptors and ecto-nucleotidases...
1999-04-01 [Naunyn Schmiedebergs Arch. Pharmacol. 359(4) , 339-44, (1999)] |