| Structure | Name/CAS No. | Articles |
|---|---|---|
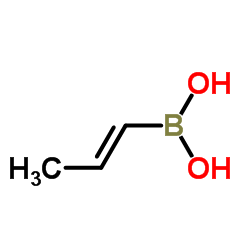 |
(1E)-1-Propen-1-ylboronic acid
CAS:7547-97-9 |
Chun-Ming Yang, Masilamani Jeganmohan, Kanniyappan Parthasarathy, Chien-Hong Cheng
Index: Org. Lett. 12 , 3610-3613, (2010)
Full Text: HTML
A highly regio- and stereoselective nickel-catalyzed three-component coupling of alkynes with enones and alkenyl boronic acids to afford highly substituted 1,3-dienes is described. The reaction can also be extended to cyclization of enynes with coupling to alkenyl boronic acids. A possible reaction mechanism involving a five-membered nickelacycle as a key intermediate is proposed.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
(1E)-1-Propen-1-ylboronic acid
CAS:7547-97-9 |
C3H7BO2 |
|
Discovery of potent, selective, and orally bioavailable alky...
2011-10-27 [J. Med. Chem. 20th ed., 54 , 7299-7317, (2011)] |
|
Selective palladium-catalyzed C-F activation/carbon-carbon b...
2012-02-17 [J. Org. Chem. 4th ed., 77 , 1798-1804, (2012)] |
|
The preparation of substituted pyrazoles from β,β-...
[Synlett 4 , 602-606, (2010)] |
|
A new strategy for the synthesis of substituted dihydropyron...
[Tetrahedron 67 , 4995-5010, (2011)] |
|
Total synthesis of rodgersinol: a survey of the Cu(II)-media...
[Tetrahedron 66 , 6826-6831, (2010)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved