Unexpected rearrangement of enantiomerically pure 3-aminoquinuclidine as a simple way of preparing diastereomeric octahydropyrrolo[2,3-c]pyridine derivatives.
Igor Goljer, Albert Molinari, Yanan He, Lisa Nogle, Weilin Sun, Brandon Campbell, Oliver McConnell
Index: Chirality 21(7) , 681-91, (2009)
Full Text: HTML
Abstract
Reaction of (S)- or (R)-3-aminoquinuclidine with 2-chloropyrimidine or 2-bromopyrimidine led to an unexpected formation of both cis- and trans-octahydropyrrolo [2,3]pyridine derivatives. A single-step synthesis of two of the four stereoisomers of these octahydropyrrolo[2,3]pyridine derivatives provides a convenient way of generating stereochemically defined isomers. Optimization of reaction conditions was carried out by (1)H NMR monitoring. The relative and absolute stereochemistry of all four stereoisomers was determined by a combination of (1)H, (13)C, and (15)N NMR spectroscopy and vibrational circular dichroism spectroscopy.2008 Wiley-Liss, Inc.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
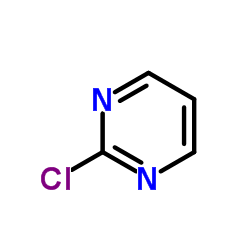 |
chloropyrimidine
CAS:1722-12-9 |
C4H3ClN2 |
|
Cobalt-catalyzed cross-coupling between in situ prepared ary...
2009-04-17 [J. Org. Chem. 74(8) , 3221-4, (2009)] |
|
Ru-catalysed C-H arylation of indoles and pyrroles with boro...
2015-03-27 [Chemistry 21(14) , 5380-6, (2015)] |
|
Expedient parallel synthesis of 2-amino-4-heteroarylpyrimidi...
2005-09-15 [Org. Lett. 7(19) , 4113-6, (2005)] |
|
Synthesis and spectral characterization of new bis(2-(pyrimi...
2012-08-01 [Arch. Pharm. (Weinheim) 345(8) , 663-9, (2012)] |
|
Synthesis and characterization of novel 2, 2'-bipyrimidine f...
2011-01-01 [Chem. Cent. J. 5 , 72, (2011)] |