Structural basis for the haemotoxicity of dapsone: the importance of the sulphonyl group.
R Mahmud, M D Tingle, J L Maggs, M T Cronin, J C Dearden, B K Park
Index: Toxicology 117(1) , 1-11, (1997)
Full Text: HTML
Abstract
The structural basis of dapsone (4,4'-diaminodiphenyl sulphone) haemotoxicity has been determined by investigation of the in vitro bioactivation of a series of 4-substituted arylamines. In the presence of rat liver microsomes, dapsone (100 microM) was the most potent former of methaemoglobin in human erythrocytes (44.8 +/- 6.7%). Substitution of the sulphone group with sulphur (11.6 +/- 1.4% methaemoglobin), oxygen (4.5 +/- 1.1%), nitrogen (0.0 +/- 3.2%), carbon (13.6 +/- 0.8%) or a keto group (34.0 +/- 6.1%) resulted in a decrease in methaemoglobin formation. Only one compound, 4,4'-diaminodiphenylamine, generated significant (P < 0.001) amounts of methaemoglobin (25.6 +/- 2.5%) in the absence of NADPH. To assess further the role of the 4-substituent in methaemoglobinaemia, the toxicity of a series of 4-substituted aniline derivatives was also studied. Of the anilines studied, 4-nitroaniline caused the most methaemoglobin (36.5 +/- 8.0%), whilst aniline caused the least (0.3 +/- 0.5%). Overall, there was a significant correlation (r2 = 0.83) between the haemotoxicity and the Hammett constant, sigma(p), suggesting that it is the electron-withdrawing properties of the substituent that influence the methaemoglobin formation. In the presence of microsomes prepared from two human livers, dapsone was the most haemotoxic bis arylamine, whereas 4-iodoaniline was the most potent methaemoglobin former (60.6 and 73.6%) and aniline the least potent (1.1 and 2.4%). As a whole, these results indicate that the sulphonyl group, which is essential for the pharmacological activity of dapsone, is also largely responsible for the haemotoxicity seen with this drug.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
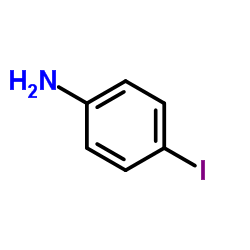 |
4-Iodoaniline
CAS:540-37-4 |
C6H6IN |
|
cIEF for rapid pKa determination of small molecules: a proof...
2014-10-15 [Eur. J. Pharm. Sci. 63 , 14-21, (2014)] |
|
Growth of Thin, Anisotropic, π-Conjugated Molecular Films by...
2015-07-15 [J. Am. Chem. Soc. 137 , 8819-28, (2015)] |
|
Potential of neurotoxicity after a single oral dose of 4-bro...
2003-01-01 [J. Appl. Toxicol. 23(5) , 315-22, (2003)] |
|
The effect of varying halogen substituent patterns on the cy...
1995-05-11 [Biochem. Pharmacol. 49(9) , 1235-48, (1995)] |
|
Selective hydrogenation of halogenated arenes using porous m...
2016-07-04 [Faraday Discuss. 188 , 451-66, (2016)] |