Enzymatic glycosylation of reducing oligosaccharides linked to a solid phase or a lipid via a cleavable squarate linker.
O Blixt, T Norberg
Index: Carbohydr. Res. 319(1-4) , 80-91, (1999)
Full Text: HTML
Abstract
Reducing oligosaccharides were converted into their corresponding glycosylamines, and these were reacted with 3,4-diethoxy-3-cyclobuten-1,2-dione (squaric acid diethyl ester). The resulting derivatives could be linked to amino-functionalized lipids, solids, or proteins. Treatment of the obtained lipid or solid conjugates with aqueous bromine or, alternatively, with ammonia-ammonium borate cleaved the linkage and regenerated the oligosaccharide glycosylamines, which were in turn rapidly hydrolyzed to the reducing oligosaccharides. To demonstrate the usefulness of this linkage in enzymatic oligosaccharide synthesis, lactose was linked to a lipid or a solid phase, the obtained conjugates were then subjected to two enzymatic glycosylations (either consecutively or 'one-pot'). The resulting materials were then cleaved to give, in both cases, the expected reducing tetrasaccharide (lacto-N-neotetraose) in good yield.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
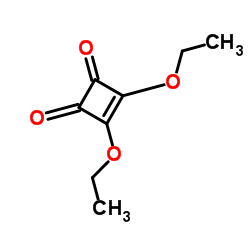 |
Diethyl squarate
CAS:5231-87-8 |
C8H10O4 |
|
[J. Org. Chem. 59 , 4707, (1994)] |
|
[Organic Synth. 69 , 220, (1990)] |
|
[J. Chem. Soc. Perkin Trans. I , 263, (1993)] |
|
Bioisosteric replacement of the alpha-amino carboxylic acid ...
1992-12-11 [J. Med. Chem. 35 , 4720, (1992)] |
|
Squaric acid mediated synthesis and biological activity of a...
2012-04-09 [Biomacromolecules 13(4) , 1161-71, (2012)] |