Squaric acid mediated synthesis and biological activity of a library of linear and hyperbranched poly(glycerol)-protein conjugates.
Frederik Wurm, Carsten Dingels, Holger Frey, Harm-Anton Klok
Index: Biomacromolecules 13(4) , 1161-71, (2012)
Full Text: HTML
Abstract
Polymer-protein conjugates generated from side chain functional synthetic polymers are attractive because they can be easily further modified with, for example, labeling groups or targeting ligands. The residue specific modification of proteins with side chain functional synthetic polymers using the traditional coupling strategies may be compromised due to the nonorthogonality of the side-chain and chain-end functional groups of the synthetic polymer, which may lead to side reactions. This study explores the feasibility of the squaric acid diethyl ester mediated coupling as an amine selective, hydroxyl tolerant, and hydrolysis insensitive route for the preparation of side-chain functional, hydroxyl-containing, polymer-protein conjugates. The hydroxyl side chain functional polymers selected for this study are a library of amine end-functional, linear, midfunctional, hyperbranched, and linear-block-hyperbranched polyglycerol (PG) copolymers. These synthetic polymers have been used to prepare a diverse library of BSA and lysozyme polymer conjugates. In addition to exploring the scope and limitations of the squaric acid diethylester-mediated coupling strategy, the use of the library of polyglycerol copolymers also allows to systematically study the influence of molecular weight and architecture of the synthetic polymer on the biological activity of the protein. Comparison of the activity of PG-lysozyme conjugates generated from relatively low molecular weight PG copolymers did not reveal any obvious structure-activity relationships. Evaluation of the activity of conjugates composed of PG copolymers with molecular weights of 10000 or 20000 g/mol, however, indicated significantly higher activities of conjugates prepared from midfunctional synthetic polymers as compared to linear polymers of similar molecular weight.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
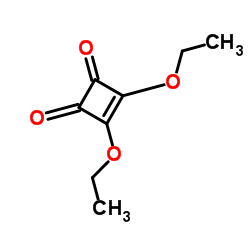 |
Diethyl squarate
CAS:5231-87-8 |
C8H10O4 |
|
[J. Org. Chem. 59 , 4707, (1994)] |
|
[Organic Synth. 69 , 220, (1990)] |
|
[J. Chem. Soc. Perkin Trans. I , 263, (1993)] |
|
Enzymatic glycosylation of reducing oligosaccharides linked ...
1999-06-30 [Carbohydr. Res. 319(1-4) , 80-91, (1999)] |
|
Bioisosteric replacement of the alpha-amino carboxylic acid ...
1992-12-11 [J. Med. Chem. 35 , 4720, (1992)] |