Synthesis and target identification of hymenialdisine analogs.
Yongqin Wan, Wooyoung Hur, Charles Y Cho, Yi Liu, Francisco J Adrian, Olivier Lozach, Stéphane Bach, Thomas Mayer, Doriano Fabbro, Laurent Meijer, Nathanael S Gray
Index: Chem. Biol. 11(2) , 247-59, (2004)
Full Text: HTML
Abstract
Hymenialdisine (HMD) is a sponge-derived natural product kinase inhibitor with nanomolar activity against CDKs, Mek1, GSK3beta, and CK1 and micromolar activity against Chk1. In order to explore the broader application of the pyrrolo[2,3-c]azepine skeleton of HMD as a general kinase inhibitory scaffold, we searched for additional protein targets using affinity chromatography in conjunction with the synthesis of diverse HMD analogs and profiled HMD against a panel of 60 recombinant enzymes. This effort has led to nanomolar to micromolar inhibitors of 11 new targets including p90RSK, KDR, c-Kit, Fes, MAPK1, PAK2, PDK1, PKCtheta, PKD2, Rsk1, and SGK. The synthesis of HMD analogs has resulted in the identification of compounds with enhanced and/or dramatically altered selectivities relative to HMD (28n) and in molecules with antiproliferative activities 30-fold higher than HMD (28p).
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
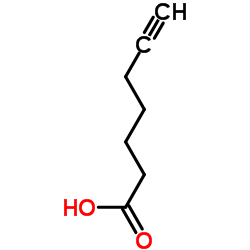 |
6-Heptynoic acid
CAS:30964-00-2 |
C7H10O2 |
|
Chemical xenobiotics and mitochondrial autoantigens in prima...
2005-05-01 [J. Immunol. 174(9) , 5874-83, (2005)] |
|
Synthesis and antiproliferative activity of some novel triaz...
2014-01-01 [Molecules 19(2) , 2523-35, (2014)] |
|
Acetylene functionalized BODIPY dyes and their application i...
2007-11-15 [Bioorg. Med. Chem. Lett. 17(22) , 6169-71, (2007)] |
|
Total synthesis of epothilones B and D.
2001-07-12 [Org. Lett. 3(14) , 2221-4, (2001)] |
|
A novel catalyst with a cuboidal PdMo3S4 core for the cycliz...
[Angew. Chem. Int. Ed. Engl. 35(18) , 2123-24, (1996)] |