6-Heptynoic acid
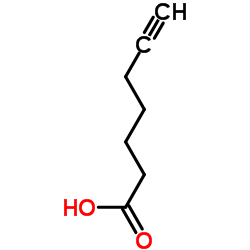
6-Heptynoic acid structure
|
Common Name | 6-Heptynoic acid | ||
|---|---|---|---|---|
| CAS Number | 30964-00-2 | Molecular Weight | 126.153 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 231.4±23.0 °C at 760 mmHg | |
| Molecular Formula | C7H10O2 | Melting Point | 22ºC | |
| MSDS | Chinese USA | Flash Point | 106.8±17.3 °C | |
| Symbol |

GHS05 |
Signal Word | Danger | |
|
Chemical xenobiotics and mitochondrial autoantigens in primary biliary cirrhosis: identification of antibodies against a common environmental, cosmetic, and food additive, 2-octynoic acid.
J. Immunol. 174(9) , 5874-83, (2005) Emerging evidence has suggested environmental factors as causative agents in the pathogenesis of primary biliary cirrhosis (PBC). We have hypothesized that in PBC the lipoyl domain of the immunodominant E2 component of pyruvate dehydrogenase (PDC-E2) is repla... |
|
|
Synthesis and antiproliferative activity of some novel triazole derivatives from dehydroabietic acid.
Molecules 19(2) , 2523-35, (2014) Dehydroabietic acid (DHA) is a naturally occurring diterpene with different and relevant biological activities. Previous studies have shown that some DHA derivatives display antiproliferative activity. However, the reported compounds did not include triazole ... |
|
|
Acetylene functionalized BODIPY dyes and their application in the synthesis of activity based proteasome probes.
Bioorg. Med. Chem. Lett. 17(22) , 6169-71, (2007) The synthesis of three acetylene functionalized BODIPY dyes is described. These dyes are used to fluorescently modify an azido functionalized epoxomicin analogue employing the Huisgen 1,3-dipolar cycloaddition, resulting in a panel of fluorescent epoxomicin d... |
|
|
Synthesis and target identification of hymenialdisine analogs.
Chem. Biol. 11(2) , 247-59, (2004) Hymenialdisine (HMD) is a sponge-derived natural product kinase inhibitor with nanomolar activity against CDKs, Mek1, GSK3beta, and CK1 and micromolar activity against Chk1. In order to explore the broader application of the pyrrolo[2,3-c]azepine skeleton of ... |
|
|
Total synthesis of epothilones B and D.
Org. Lett. 3(14) , 2221-4, (2001) [reaction: see text] A highly convergent total synthesis of the natural products epothilone B and D is described. The route is highlighted by efficient generation of a C12-C13 trisubstituted olefin which exploits a sequential Nozaki-Hiyama-Kishi coupling and ... |
|
|
A novel catalyst with a cuboidal PdMo3S4 core for the cyclization of alkynoic acids to enol lactones. Wakabayashi T, et al.
Angew. Chem. Int. Ed. Engl. 35(18) , 2123-24, (1996)
|