A phase II trial of ilmofosine in non-small cell bronchogenic carcinoma.
P V Woolley, C J Schultz, G I Rodriguez, R A Gams, K W Rowe, M L Dadey, D D Von Hoff, J J McPhillips
Index: Invest. New Drugs 14(2) , 219-22, (1996)
Full Text: HTML
Abstract
We have conducted a study of ilmofosine (1-hexadecylthio; 2-methoxyethyl-rac-glycero-3-phosphocholine) in non-small cell bronchogenic carcinoma, using a schedule of continuous infusion for 5 days and a dose of 300 mg/m2/day. Toxicities were gastrointestinal (nausea, vomiting, diarrhea), fatigue and liver function abnormalities. These were severe and resulted in the removal of some patients from study. No consistent pattern of bone marrow suppression was seen. No tumor regressions occurred in 14 evaluable patients including 5 with no prior therapy. We conclude that ilmofosine is inactive in this tumor at this dose and schedule.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
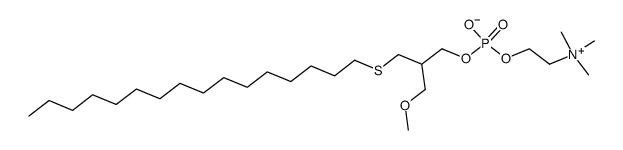 |
ILMOFOSINE
CAS:83519-04-4 |
C26H56NO5PS |
|
Phase I trial of ilmofosine as a 24 hour infusion weekly.
2010-01-01 [Invest. New Drugs 13(3) , 205-10, (1995)] |
|
Structure-function relationships of alkyl-lysophospholipid a...
1993-06-01 [Lipids 28(6) , 511-6, (1993)] |
|
Quaternary ammonium analogs of ether lipids inhibit the acti...
1998-01-01 [Cancer Chemother. Pharmacol. 42(4) , 319-26, (1998)] |
|
Expression of two 50 kDa proteins is associated with sensiti...
1997-10-01 [Eur. J. Cancer 33(11) , 1875-80, (1997)] |
|
Cytotoxicity of ether phospholipid BM 41.440 on neuroblastom...
1995-01-01 [J. Cancer Res. Clin. Oncol. 121(5) , 262-6, (1995)] |