Effect of a p-nitro group of phenyl-maltooligosaccharide substrate on the change of action specificity of lysine-modified porcine pancreatic alpha-amylase.
H Yamashita, H Nakatani, B Tonomura
Index: Biochem. Mol. Biol. Int. 35(1) , 79-85, (1995)
Full Text: HTML
Abstract
The effect of chemical modification of lysine residues on the activity of porcine pancreatic alpha-amylase (PPA) was examined, using p-nitrophenyl-alpha-D-maltoside, p-nitrophenyl-alpha-D-maltotrioside, phenyl-alpha-D-maltoside and phenyl-alpha-D-maltotrioside as substrates. Chemical modification of PPA with trinitrobenzenesulfonic acid enhanced the kcat/Km values for p-nitrophenyl substrates, but not for phenyl substrates. Thus, this effect is substituent selective. Considering the productive binding modes of substrates to PPA, the p-nitro group of the substrate and the modified lysine residues of the enzyme would non-ionically interact with each other to stabilize the productive binding mode.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
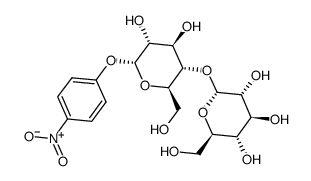 |
P-NITROPHENYL-ALPHA-D-MALTOSIDE
CAS:17400-77-0 |
C18H25NO13 |
|
Kinetic characterization of glycosidase activity from disacc...
2005-05-01 [J. Pharm. Pharmacol. 57(5) , 661-4, (2005)] |
|
Rapid determination of alpha-amylase activity by use of a ne...
1987-04-01 [Clin. Chem. 33(4) , 524-8, (1987)] |
|
Barley malt-alpha-amylase. Purification, action pattern, and...
1992-09-23 [Biochim. Biophys. Acta 1159(2) , 193-202, (1992)] |
|
Differential rate assay of human pancreatic and salivary alp...
1986-11-01 [J. Biochem. 100(5) , 1353-8, (1986)] |
|
Differentiation of human non-salivary, non-pancreatic alpha-...
1990-04-01 [J. Biochem. 107(4) , 546-9, (1990)] |