33538-71-5
| Name | venturicidin A |
|---|---|
| Synonyms |
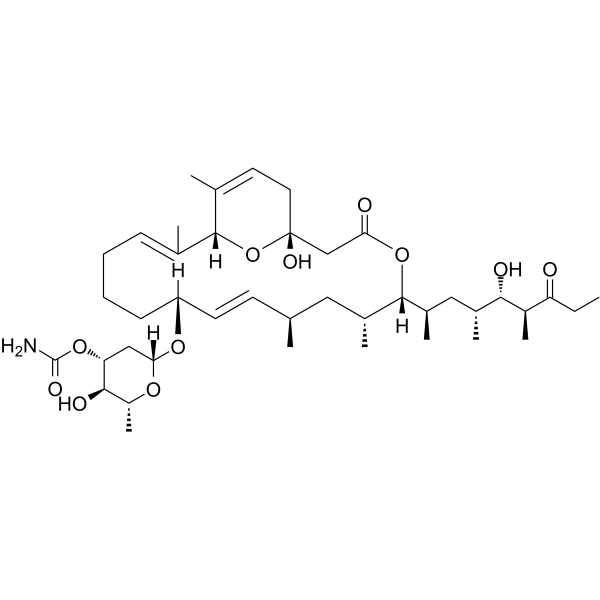
(1R,5S,6R,8R,9E,11R,15E,17R)-1-Hydroxy-5-[(2R,4R,5S,6S)-5-hydroxy-4,6-dimethyl-7-oxononan-2-yl]-6,8,16,18-tetramethyl-3-oxo-4,21-dioxabicyclo[15.3.1]henicosa-9,15,18-trien-11-yl 3-O-carbamoyl-2,6-dideoxy-β-D-arabino-hexopyranoside
Venturicidin A (1R,5S,6R,8R,9E,11R,15E,17R)-1-Hydroxy-5-[(2R,4R,5S,6S)-5-hydroxy-4,6-dimethyl-7-oxo-2-nonanyl]-6,8,16,18-tetramethyl-3-oxo-4,21-dioxabicyclo[15.3.1]henicosa-9,15,18-trien-11-yl 3-O-carbamoyl-2,6-dide ;oxy-β-D-arabino-hexopyranoside venturicidin a (aabomycin a1) |
| Description | Venturicidin A (Aabomycin A1), from actinomycetes, is a membrane-active natural product inhibitor of ATP synthase. Venturicidin A potentiates the aminoglycoside antibiotic gentamicin against multidrug-resistant clinical isolates of Staphylococcus, Enterococcus, and Pseudomonas aeruginosa. Venturicidin A shows noticeable toxicity toward human embryonic-kidney (HEK)cells with an IC50 of 31 μg/mL[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 874.9±65.0 °C at 760 mmHg |
| Molecular Formula | C41H67NO11 |
| Molecular Weight | 749.971 |
| Flash Point | 482.9±34.3 °C |
| Exact Mass | 749.471436 |
| PSA | 184.07000 |
| LogP | 5.54 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.541 |