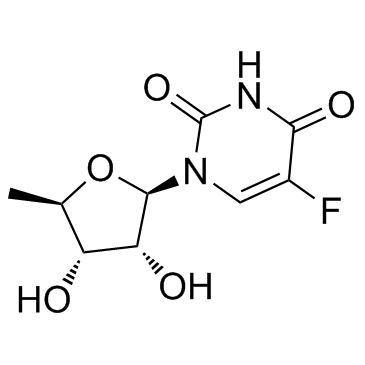
3094-09-5
| Name | doxifluridine |
|---|---|
| Synonyms |
5-Fluorodesoxyuridine
RO-21-9738 EINECS 221-440-1 doxifluridine 5'DFURD Flutron Furtulon 1-(5-Deoxy-β-D-ribofuranosyl)-5-fluorouracil 5'-DFUR doxyfluridine 1-(b-D-5'-Deoxyribofuranosyl)-5-fluorouracil 5-Fluoro-5'-deoxyuridine 5'-Deoxy-5-fluorouridine MFCD00866530 5'-dFUrd 5'-DEOXYFLUOROURIDINE (+)-5-Fluoro-5'-deoxyuridine Doxifluidine DOXIFLUIRDINE |
| Description | Doxifluridine(Ro 21-9738; 5'-DFUR) is a thymidine phosphorylase activator for PC9-DPE2 cells with IC50 of 0.62 μM. IC50 value: 0.62 μM(PC9-DPE2 cell).Target: Nucleoside antimetabolite/analogDoxifluridine is a fluoropyrimidine derivative and oral prodrug of the antineoplastic agent 5-fluorouracil (5-FU) with antitumor activity. Doxifluridine, designed to circumvent the rapid degradation of 5-FU by dihydropyrimidine dehydrogenase in the gut wall, is converted into 5-FU in the presence of pyrimidine nucleoside phosphorylase. 5-FU interferes with DNA synthesis and subsequent cell division by reducing normal thymidine production and interferes with RNA transcription by competing with uridine triphosphate for incorporation into the RNA strand.in vitro: 5'-DFUR's metabolic product(N3-Me-5'-dFUR) was found to be non-toxic in all the cell growth experiments performed. The absence of cytotoxicity could be explained by the observation that the metabolite was not recognized as a substrate by thymidine phosphorilase, the enzyme responsible for 5-fluorouracil (5-FU) release from doxifluridine, as ascertained by high-performance liquid chromatography/ultraviolet (HPLC-UV) analysis of the incubation mixture[1].in vivo: Administration of 200 mg of Furtulon to 23 beagle dogs, the plasma concentrations of doxifluridine, 5-FU, and 5-FUrd were measured simultaneously, using LC-MS/MS. The parent-metabolite compartment model with first-order absorption and Michaelis-Menten kinetics described the pharmacokinetics of doxifluridine, 5-FU, and 5-FUrd. Michaelis-Menten kinetics sufficiently explained the generation and elimination processes of 5-FU and 5-FUrd[2].Clinical trial: A phase II study of doxifluridine and docetaxel combination chemotherapy for advanced or recurrent gastric cancer was reported in 2009[3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Melting Point | 188-192 °C(lit.) |
| Molecular Formula | C9H11FN2O5 |
| Molecular Weight | 246.192 |
| Exact Mass | 246.065201 |
| PSA | 104.55000 |
| LogP | -0.96 |
| Index of Refraction | 1.605 |
| Storage condition | -20°C Freezer |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | YU7526000 |
| HS Code | 2934999090 |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |