84633-29-4
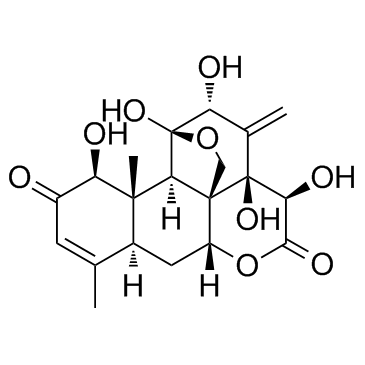
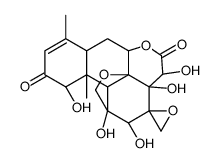
| Name | eurycomanone |
|---|---|
| Synonyms |
(1β,11α,12α,15β)-1,11,12,14,15-Pentahydroxy-11,20-epoxypicrasa-3,13(21)-diene-2,16-dione dihydrate
pasakbumin-a EURYCOMANONE Picrasa-3,13(21)-diene-2,16-dione, 11,20-epoxy-1,11,12,14,15-pentahydroxy-, (1β,11α,12α,15β)-, hydrate (1:2) |
| Description | Eurycomanone could increases spermatogenesis by inhibiting the activity of phosphodiesterase and aromatase in steroidogenesis. |
|---|---|
| Related Catalog | |
| In Vitro | Eurycomanone (EN) significantly increased testosterone production dose-dependently at 0.1, 1.0 and 10.0 μM, but the two lower doses when combined with 3-isobutyl-1-methylxanthine (IBMX), the phosphodiesterase inhibitor were not significantly higher than EN or IBMX alone, except at a higher concentration. The molecular docking studies indicated EN and IBMX were binding at different sites of the enzyme. EN has no reversal of inhibition by aminoglutethimide, ketoconazole or nifedipine at the respective steroid oogenesis enzyme. The quassinoid was also non-responsive to the inhibition of oestrogen receptor by tamoxifen, but displayed improved for mestane inhibition of aromatase in reducing oestrogen production. The molecular docking studies further supported that EN and formestane bound to aromatase with similar orientations and free energy binding values[1]. |
| References |
| Melting Point | 273-285℃ (methanol ethyl ether ) |
|---|---|
| Molecular Formula | C20H24O9 |
| Molecular Weight | 408.40 |
| PSA | 172.21000 |
| Storage condition | 2-8℃ |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Precursor 0 | |
|---|---|
| DownStream 1 | |