500-92-5
| Name | proguanil |
|---|---|
| Synonyms |
bigumal
chloroguanide N-(4-CHLOROPHENYL)-N'-(ISOPROPYL)-IMIDODICARBONIMIDIC DIAMIDE EINECS 207-915-6 Chlorguanide-D6Cl PROGUANIL chlorguanide paludrine ChlorguanideCl |
| Description | Proguanil is an antimalarial prodrug that is metabolized to the active metabolite cycloguanil, a dihydrofolate reductase (DHFR) inhibitor. |
|---|---|
| Related Catalog | |
| In Vitro | Proguanil per se has only weak antimalarial activity in vitro (IC50=2.4-19 μM), and its effectiveness depends on the active metabolite cycloguanil (IC50=0.5-2.5 nM). The cycloguanil is a dihydrofolate reductase (DHFR) inhibitor. The combination of atovaquone and proguanil is synergistic in vitro. Both drugs also have activity against gametocytes and pre-erythrocytic (hepatic) stages of malaria parasites[1]. Proguanil acts as a biguanide rather than as its metabolite cycloguanil (a parasite dihydrofolate reductase [DHFR] inhibitor) to enhance the atovaquone effect; proguanil-mediated enhancement is specific for atovaquone, since the effects of other mitochondrial electron transport inhibitors, such as myxothiazole and antimycin, are not altered by inclusion of proguanil[2]. 5-HT3 receptor responses are reversibly inhibited by proguanil, the metabolite 4-chlorophenyl-1-biguanide (CPB) and the active metabolite cycloguanil (CG), with an IC50 of 1.81, 1.48 and 4.36 μM, respectively[3]. |
| In Vivo | Proguanil could induce infertility in rats which may act by distorting the blood-testis barrier formed by the Sertoli cells. Duration dependent significant decrease in body and organ weights and also in sperm parameters is observed. Serum testosterone level is significantly decreased for proguanil treatment rats[4]. Administration of Malarone (atovaquone and proguanil) to experimentally B. gibsoni infected two dogs in chronic stage and three dogs in acute stage results in decrease in parasitemia, and clinical improvements are observed[5]. |
| Cell Assay | Sertoli cells obtained from sixteen to eighteen day-old-rats are cultured and treated with 0.3 μM to 10 μM of proguanil for 5 days after which Sertoli cell viability and nuclei integrity are determined. Also, the genetic expressions of transferrin and Glial cell line-derived neurotrophic factor are assessed[4]. |
| Animal Admin | Rats: Groups of ten to twelve-week-old rats are administered proguanil (2.9 mg/kg body weight) daily for 5 days and 6 weeks respectively. Thereafter, body and reproductive organ weights are taken, sperm parameters are analyzed, while the histology of the testis and epididymis are carried out. Also, serum levels of testosterone, luteinizing hormone and follicle stimulating hormone are determined[4]. |
| References |
| Density | 1.29g/cm3 |
|---|---|
| Boiling Point | 402.7ºC at 760mmHg |
| Melting Point | 129° |
| Molecular Formula | C11H16ClN5 |
| Molecular Weight | 253.73100 |
| Flash Point | 197.4ºC |
| Exact Mass | 253.10900 |
| PSA | 83.79000 |
| LogP | 3.26330 |
| Index of Refraction | 1.6110 (estimate) |
| Storage condition | 2-8℃ |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
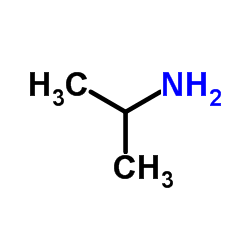
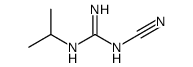
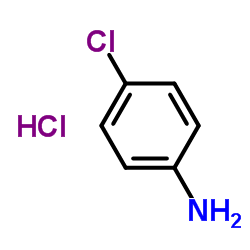
| Precursor 9 | |
|---|---|
| DownStream 0 | |


![3-[(4-chlorophenyl)thiocarbamoyl]-1-propan-2-yl-thiourea structure](https://image.chemsrc.com/caspic/346/79491-01-3.png)